Aortic stenosis (AS) is a significant obstructive valvular disorder including degenerative, bicuspid and rheumatic aetiologies.1 While the true disease burden of AS in the Asia-Pacific region is unknown, the ageing population across the region and the high prevalence of bicuspid aortic valves (AVs) render AS a potentially growing problem.
Transcatheter AV implantation (TAVI) has been established as an effective treatment modality in patients with severe AS and the adoption of TAVI is rapidly growing in the Asia-Pacific region.1 Results from the region have been comparable with international outcomes, with the Asia Pacific TAVI registry, a multicentre, prospective observational registry, reporting a 97.5% 30-day survival in 1,125 patients treated with TAVI.1 However, there is heterogeneity in the management of AS and the use of TAVI between the Asia-Pacific and Western regions. Reasons for these differences include anatomical variations, substantial disparity in healthcare resources and infrastructure and the lack of consensus on the optimal management of AS in the Asia-Pacific region.
Given these challenges, there is a need for unified, practical guidance on the matter. Hence, the Asian Pacific Society of Cardiology (APSC) developed these recommendations focusing on the appropriate management of AS and role of TAVI. These position statements were developed with general cardiologists and internal medicine specialists practising cardiology as the intended readers.
Methods
The APSC convened an expert panel to review the literature on the diagnosis and management of AS, discussed gaps in current management, determined areas where further guidance is needed, and developed recommendations on the assessment, referral and management of patients with AS. The 32 experts on the panel, comprising a multidisciplinary group of general and interventional cardiologists, cardiac surgeons and imaging specialists, are members of the APSC who were nominated by national societies and endorsed by the APSC consensus board, or invited international experts.
After a comprehensive literature search, selected applicable articles were reviewed and appraised using the Grading of Recommendations Assessment, Development, and Evaluation system.2 Based on this system, the levels of evidence were designated as follows:
- High (authors have high confidence that the true effect is similar to the estimated effect).
- Moderate (authors believe that the true effect is probably close to the estimated effect).
- Low (true effect might be markedly different from the estimated effect).
- Very low (true effect is probably markedly different from the estimated effect).
As indicated in these levels, the authors adjusted the level of evidence if the estimated effect, when applied in the Asia-Pacific region, may differ from the published evidence because of various factors such as ethnicity, cultural differences and/or healthcare systems and resources.
The available evidence was discussed and a set of recommendations were developed during a virtual consensus meeting in August 2023 and a face-to-face meeting in October 2023. The final position statements were then put to an online vote, with each recommendation being voted on by each panel member using a three-point scale (i.e. agree, neutral or disagree). Consensus was reached when 80% of votes for a recommendation were ‘agree’ or ‘neutral’. In the case of non-agreement, the recommendations were further discussed using email communication and then revised accordingly until the criteria for consensus were fulfilled.
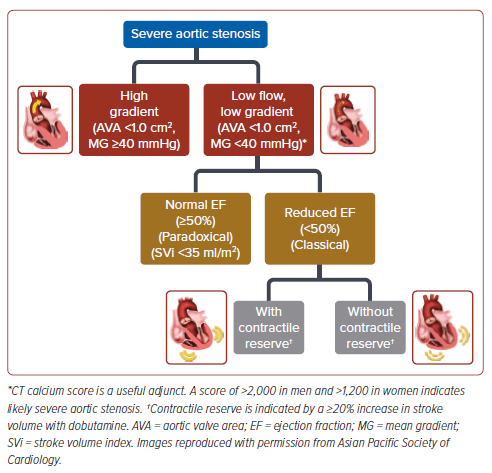
For these position statements, the authors made adaptations to the classification of AS severity of the 2020 American College of Cardiology/ American Heart Association (ACC/AHA) guidelines and the 2021 European Society of Cardiology (ESC) guidelines.3,4 Mild AS was defined as AS with an aortic maximum velocity (Vmax) of 2.0–2.9 m/s or a mean aortic pressure gradient (MG) of <20 mmHg and an aortic valve area (AVA) of ≥1.5 cm2 . Moderate AS was defined as an aortic Vmax of 3.0–3.9 m/s or an aortic MG of 25–40 mmHg and AVA of 1.0–1.5 cm2 . Severe AS was defined as an aortic Vmax of ≥4.0 m/s or an aortic MG of ≥40 mmHg and an AVA of ≤1.0 cm2 . The various types of severe AS are described in Figure 1.
Recommendations
Frequency of Echocardiography FollowUp of Patients with Aortic Stenosis
Statement 1. Follow-up echocardiography should be performed every 3–5 years for patients with mild AS.
Level of evidence: Low.
Level of consensus: 89.3% agree; 10.7% neutral; 0% disagree.
Statement 2. Follow-up echocardiography should be performed every 6 months to 1 year for patients with moderate AS.
Level of evidence: Low.
Level of consensus: 82.1% agree; 17.9% neutral; 0% disagree.
Statement 3. Earlier follow-up echocardiography should be performed in patients with moderate AS at higher risk of progression (e.g. severely calcified valve).
Level of evidence: Low.
Level of consensus: 96.4% agree; 3.6% neutral; 0% disagree.
Statement 4. Follow-up echocardiography should be performed every 6 months to 1 year for asymptomatic patients with severe AS.
Level of evidence: Low.
Level of consensus: 92.8% agree; 3.6% neutral; 3.6% disagree.
Statement 5. Earlier follow-up echocardiography should be performed in patients with asymptomatic severe AS at higher risk of adverse events (e.g. severely calcified valve, impaired left ventricular ejection fraction [LVEF]).
Level of evidence: Low.
Level of consensus: 96.4% agree; 0% neutral; 3.6% disagree.
AS is a progressive disease. In addition to routine clinical follow-up with history and physical examination, echocardiography is recommended to monitor disease progression by evaluating changes in valve anatomy, haemodynamics and systolic dysfunction among others.3 The 2020 ACC/ AHA guidelines recommend surveillance every 3–5 years in patients with mild AS.3 Given the lack of Asian data on the natural history of mild AS, the consensus panel generally agrees with this international recommendation. For patients with moderate AS or asymptomatic severe AS, a 6-month to 1-year frequency of echocardiographic surveillance was proposed. During the panel discussion, the authors also shared that adherence to patient follow-up is low, especially among asymptomatic patients. This potential delay should be accounted for when scheduling surveillance visits. The authors also agreed that certain at-risk patients, such as those with severely calcified AVs and those with impaired LVEF, may require more frequent surveillance due to the possibility of rapid progression and the potential benefit of early intervention.
Asymptomatic Aortic Stenosis Patients
Statement 6. Intervention is recommended in asymptomatic patients with severe AS and systolic left ventricular dysfunction (LVEF <50%).
Level of evidence: High.
Level of consensus: 100% agree; 0% neutral; 0% disagree.
Statement 7. Intervention should be considered in asymptomatic patients with severe AS who develop symptoms or a sustained fall in blood pressure (>20 mmHg) during exercise testing.
Level of evidence: Moderate.
Level of consensus: 100% agree; 0% neutral; 0% disagree.
Statement 8. Intervention should be considered in asymptomatic patients with severe AS if the procedural risk is low and one of the following parameters is present:
1. very severe AS (MG ≥60 mmHg or Vmax ≥5 m/s)
2. rapid disease progression (Vmax progression ≥0.3 m/s/year).
Level of evidence: Moderate.
Level of consensus: 91.7% agree; 8.3% neutral; 0% disagree.
The consensus panel agreed on a certain subset of asymptomatic patients with severe AS in whom intervention is recommended or should be considered. In asymptomatic patients with severe AS, intervention is recommended if LVEF is <50% (not due to other reasons) because of evidence of improved outcomes compared with medical treatment alone.5–8 Asymptomatic patients with severe AS should also receive intervention if symptoms or a blood pressure fall of >20 mmHg (ESC)/>10 mmHg (ACC/AHA) develop during exercise testing.3,9–12
For those asymptomatic patients with low procedural risk and either critical AS (MG ≥60 mmHg or Vmax ≥5 m/s) or rapid disease progression (Vmax progression ≥0.3 m/s/year), intervention should be considered.6,13–15 The rate of cardiovascular mortality in these patients is high without intervention and a majority develop symptoms within 2 years. Studies have shown that these patients may benefit from early intervention compared with conservative treatment.4 Another consideration for intervention is in those with low procedural risk and elevated brain natriuretic peptide (>3 times normal).
Symptomatic Aortic Stenosis Patients
Statement 9. Intervention is recommended in symptomatic patients with severe, high-gradient AS (MG ≥40 mmHg, Vmax ≥4.0 m/s and valve area ≤1.0 cm2 [or ≤0.6 cm2 /m2 ]).
Level of evidence: High.
Level of consensus: 89.3% agree; 10.7% neutral; 0% disagree.
Statement 10. Intervention is recommended in symptomatic patients with true severe low-flow, low-gradient (<40 mmHg) AS with reduced ejection fraction (<50%), and evidence of contractile reserve (stroke volume improvement ≥20%, Vmax ≥4.0 m/s, valve area ≤1.0 cm2 with dobutamine).
Level of evidence: High.
Level of consensus: 89.3% agree; 10.7% neutral; 0% disagree.
Statement 11. Intervention should be considered in symptomatic patients with low-flow (stroke volume index [SVi] ≤35 ml/m2 ), lowgradient (<40 mmHg) AS with normal ejection fraction after confirmation that the AS is severe.
Level of evidence: Moderate.
Level of consensus: 92.9% agree; 7.1% neutral; 0% disagree.
Statement 12. Intervention should be considered in symptomatic patients with low-flow, low-gradient severe AS and reduced ejection fraction without flow (contractile) reserve, particularly when CT Agatston calcium scoring (male >2,000, female >1,200) indicates severe AS.
Level of evidence: Moderate.
Level of consensus: 96.4% agree; 3.6% neutral; 0% disagree.
Statement 13. Intervention is not recommended in patients with symptomatic AS with severe comorbidities when the intervention is unlikely to improve quality of life or prolong survival >1 year.
Level of Evidence: Very low.
Level of consensus: 96.4% agree; 3.6% neutral; 0% disagree.
The consensus panel agreed that intervention is recommended in symptomatic patients with severe AS with the following characteristics: either high-gradient AS (MG ≥40 mmHg, peak aortic velocity ≥4.0 m/s, and AVA ≤1.0 cm2 [or ≤0.6 cm2 /m2 ]); or low-flow, low-gradient AS with reduced LVEF (<50%) and evidence of true AS with contractile reserve (stroke volume improvement ≥20%, peak aortic velocity ≥4.0 m/s, AVA ≤1.0 cm2 with dobutamine).15–21 The benefit of intervention in these patients is well established.
Furthermore, intervention should also be considered in symptomatic patients with low-flow (SVi ≤35 ml/m2 ), low-gradient (<40 mmHg) severe AS with normal LVEF (i.e., paradoxical low-flow AS).22–24 While some benefit from intervention has been demonstrated in these patients, there is some uncertainty in the benefit as evidenced by a wide confidence interval shown in a network meta-analysis.25
In addition, intervention should be considered in patients with low-flow, low-gradient severe AS and reduced LVEF without flow (contractile) reserve, particularly when CT Agatston calcium scoring (males >2,000, females >1,200) indicates severe AS. While outcomes in these patients are generally poor even with intervention, some evidence suggests that, even though there is an increased risk of procedural mortality, intervention does improve LVEF and clinical outcomes.26–28
The authors highlight that in a low-flow, low-gradient situation, true severe AS should be differentiated from pseudo-severe AS (wherein patients have only moderate AS with incomplete valve opening, usually due to accompanying myocardial dysfunction). Low-dose dobutamine stress echocardiography (DSE) may be used to differentiate true- versus pseudo-severe AS.29 With true-severe AS and contractile reserve (stroke volume improvement ≥20% with dobutamine), there will be little or no increase in AVA and a significant increase in gradient, which is congruent with the relative increase in flow. In contrast, with pseudo-severe AS, there will be an increase in AVA and little or no increase in gradient. Calcium scoring using multidetector CT is useful to corroborate AS severity when DSE is not feasible or not conclusive, especially in the cases without contractile reserve, with scores of >2,000 in males and >1,200 in females indicating likely severe AS.30,31
Lastly, the consensus panel agreed that intervention is not recommended in patients with symptomatic AS with severe comorbidities when the intervention is unlikely to improve quality of life or prolong survival >1 year. In this situation, the risks of intervention will likely outweigh its benefits.
Transcatheter Aortic Valve Implantation Versus Surgical Aortic Valve Replacement
Statement 14. The choice between surgical AV replacement (SAVR) and TAVI should be based on careful evaluation of the clinical, anatomical, and procedural factors by the Heart Team.
Level of evidence: Low.
Level of consensus: 100% agree; 0% neutral; 0% disagree.
Statement 15. The lifetime management strategy for each patient should be considered and individualised.
Level of evidence: Low.
Level of consensus: 100% agree; 0% neutral; 0% disagree.
Statement 16. The Heart Team recommendation should be discussed with the patient for an informed, shared decision-making treatment choice.
Level of evidence: Very low.
Level of consensus: 100% agree; 0% neutral; 0% disagree.
Statement 17. TAVI is recommended in older patients (≥75 years) or those at high surgical risk (Society of Thoracic Surgeons [STS] Predicted Risk Of Mortality score [STSPROM]/European System for Cardiac Operative Risk Evaluation II [EuroSCORE II] >8% or Heart Team assessment).
Level of evidence: High.
Level of consensus: 92.9% agree; 7.1% neutral; 0% disagree.
Statement 18. In younger patients (65–75 years) at low/intermediate surgical risk (STSPROM/EuroSCORE II <8%), the treatment approach should be a shared decision between the patient and the Heart Team.
Level of evidence: Low.
Level of consensus: 100% agree; 0% neutral; 0% disagree.
Statement 19. In patients younger than 65 years, surgical intervention is recommended unless the patient is deemed high risk for surgery by the Heart Team.
Level of evidence: High.
Level of consensus: 82.1% agree; 14.3% neutral; 3.6% disagree.
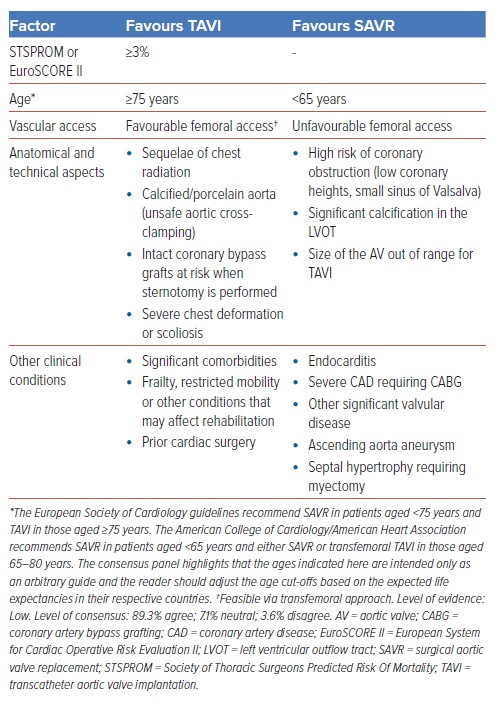
Patients with AS currently have surgical and transcatheter options for valve replacement, with each intervention carrying its inherent benefits and risks. The patient’s clinical characteristics play an important role in the decision-making process. Hence, the choice between SAVR and TAVI should be based on careful evaluation of the clinical, anatomical and procedural factors by the Heart Team. The Heart Team recommendation should be discussed with the patient for an informed and shared treatment decision. The consensus panel shares a simple guide to aid in the decision on the best treatment approach for AS in Table 1.
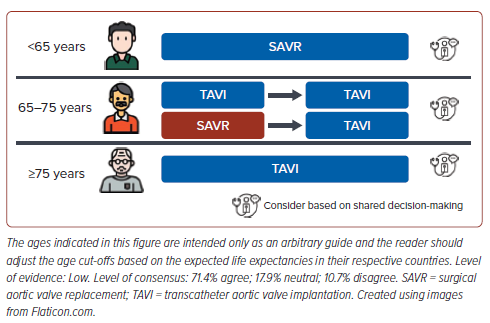
Patients with AS may potentially require multiple interventions throughout their lifetime, especially with the degeneration of bioprosthetic valves over time in younger patients. Hence, the lifetime management of the patient with AS (i.e., the optimal sequence of potential treatment strategies the patient may need to undergo over their lifetime) should be carefully considered, especially when deciding the first intervention. Patient–prosthesis mismatch, coronary re-access and the risk of conduction disturbances should all be considered in decision-making in relative younger patients. A proposed approach is shown in Figure 2. The ages indicated in Figure 2 are intended only as an arbitrary guide and the reader should adjust the age cut-offs based on the expected life expectancies in their respective countries.
There is consensus that patients younger than 65 years should undergo SAVR as the initial intervention (Figure 2) with consideration of mechanical AV replacement as bioprosthetic AV replacement might not have significantly longer durability compared with TAVI. This is due to the expected longer life expectancy at this younger age and thus the need for an option with longer durability to minimise the need for future reintervention. While more contemporary data are emerging, there are currently limited data on the very long term durability of TAVI in the younger age group. Beyond age, there are other clinical factors (e.g. comorbidities, anatomical challenges) to consider and thus shared decision-making on the optimal therapy between the Heart Team and patient is paramount.
In patients aged 65–75 years, both SAVR and TAVI are reasonable initial approaches, but due consideration should be given to the possibility of a repeat intervention in the future should the initial valve degenerate. In this instance, the likely repeat intervention, if anatomically suitable, should be TAVI. There is established data for TAV-in-SAV and the field of TAV-in-TAV is growing with increasing experience.32,33 The role of CT in planning such repeat interventions is crucial, but details of this are beyond the scope of this discussion. In patients aged ≥75 years, TAVI as the initial intervention was deemed reasonable by the consensus group.
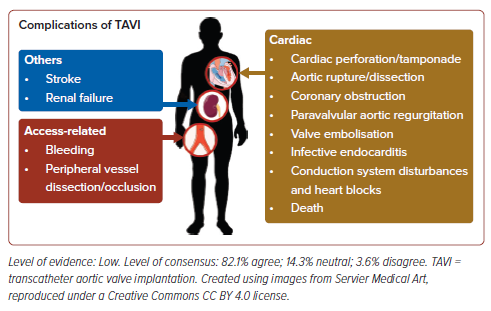
Lastly, the authors note that TAVI, while safe and efficacious, may have several complications that general cardiologists should be aware of. These complications are summarised in Figure 3.
Preprocedural Evaluation
Statement 20. Prior to TAVI, CT is recommended as the preferred imaging tool for procedural planning.
Level of evidence: Moderate.
Level of consensus: 100% agree; 0% neutral; 0% disagree.
CT is useful in the evaluation of anatomy of the AV, aorta and peripheral vessels, as well as in aiding the assessment of the severity of AS. As previously mentioned, quantitation of AV calcium by CT imaging assists in the determination of the severity of AS, with scores of >2,000 in males and >1,200 in females indicating likely severe AS. Importantly, CT imaging assists in the preprocedural planning of patients undergoing TAVI. CT provides accurate measurements of annulus area, leaflet length, coronary heights and dimensions of the aortic complex.3 CT imaging of the peripheral vessels allows careful assessment of the size, calcifications and tortuosity of the peripheral vessels to best determine the access routes and any potential difficulties.34,35 The role of CT planning prior to SAVR, while not uniformly practiced, may be a useful adjunctive tool to consider. In patients with advanced kidney disease, the risk and benefit of a CT should be weighed. If unable to perform CT, cardiac imaging using 3D transoesophageal echocardiogram and peripheral imaging with duplex ultrasound may be considered.34,36
CT imaging may also detect concomitant coronary artery disease (CAD); however, there is a lack of strong evidence to recommend its routine use for this purpose and there may be challenges to the accuracy in the setting of high coronary calcifications.
Revascularisation in Patients with Pre-existing CAD
Statement 21. In patients undergoing TAVI with significant left main or proximal CAD, revascularisation by percutaneous coronary intervention (PCI) before TAVI may be considered.
Level of evidence: Low.
Level of consensus: 89.3% agree; 10.7% neutral; 0% disagree.
A substantial number of patients with severe AS (24–45%) have concomitant CAD, in part due to the increased prevalence with advancing age.37 A meta-analysis of non-randomised studies found that, while 30- day all-cause mortality was similar between patients undergoing TAVI with or without CAD, 1-year mortality was higher in patients with CAD.38
The clinical consensus statement from the European Association of Percutaneous Cardiovascular Interventions in collaboration with the ESC Working Group on Cardiovascular Surgery on the management of CAD in patients undergoing TAVI recommended that PCI before TAVI should be performed in patients with severe CAD (i.e., coronary artery diameter stenosis >70%; >50% for the left main) only in proximal segments, particularly if presenting with an acute coronary syndrome, symptoms of angina pectoris or sub-occlusive lesions (i.e., >90% diameter stenosis).39 The consensus statement also noted that the timing of PCI regarding the TAVI procedure should be based on clinical presentation, the patient’s anatomical characteristics and coronary lesion complexity.
The consensus panel agreed to weakly recommend revascularisation prior to TAVI in patients with CAD and limited the recommendation to those with significant CAD or proximal lesions as randomised controlled trial data are not available. While the timing of revascularisation is not well established, PCI before TAVI offers several benefits over the converse, including easier coronary access, especially for self-expanding transcatheter heart valves with a supra-annular leaflet position, and a lower risk of ischaemia-induced haemodynamic instability during the TAVI procedure.39 However, this is counter-balanced by the potential increased risk of PCI in the setting of severe AS. A large non-randomised registry study showed that PCI post-TAVI seemed to be associated with improved 2-year clinical outcomes compared with other timings, but it was caveated that the results needed to be validated in randomised trials.40
Procedural Techniques
While the exact procedural techniques are not the focus of this consensus document, several general pointers may be considered:
- Standard fluoroscopic views should be employed during valve deployment. The use of cusp-overlap view where appropriate may be considered, in particular for self-expanding valves.
- The use of commissural-alignment techniques where appropriate should be considered, in particular for self-expanding valves to facilitate future coronary access/valve re-intervention.
- Where there is a higher risk of coronary occlusion and SAVR is not a feasible option, mitigation measures including upfront coronary protection should be considered. Where appropriate and expertise available, adjunct procedures such as leaflet modification (BASILICA) may be considered.
- There is currently insufficient evidence to recommend for or against the routine use of cerebral embolic protection in patients undergoing TAVI. Individualised use of cerebral embolic protection in selected patients based on a centre’s experience and practice may be reasonable.
Antithrombotic Therapy after TAVI
Statement 22. Lifelong single antiplatelet therapy (SAPT) is recommended after TAVI in patients with no baseline indication for oral anticoagulants (OAC).
Level of evidence: High.
Level of consensus: 82.1% agree; 17.9% neutral; 0% disagree.
Statement 23. Routine use of OACs is not recommended after TAVI in patients with no baseline indication for OACs.
Level of evidence: High.
Level of consensus: 92.8% agree; 3.6% neutral; 3.6% disagree.
Statement 24. OACs are recommended for TAVI patients who have other lifelong indications for OACs.
Level of evidence: High.
Level of consensus: 92.9% agree; 7.1% neutral; 0% disagree.
The American and European guidelines both consider SAPT post-TAVI as appropriate for patients without indications for OACs, based on evidence that dual antiplatelet therapy is associated with an increased risk of bleeding compared with SAPT.3,4,41,42 Similarly, the routine use of OAC is not recommended because of the results of the GALILEO study indicating a higher risk of death or thromboembolic complications and a higher risk of bleeding with a rivaroxaban-based therapy than an antiplatelet-based strategy.41 In patients with indications for anticoagulation, OACs (without antiplatelets) alone may be given lifelong post-TAVI. Data from the POPular TAVI trial showed that the addition of clopidogrel increased the 1-year incidence of serious bleeding compared with OAC alone.42
Special Scenarios
Statement 25. TAVI may be considered in anatomically suitable patients with bicuspid AV.
Level of evidence: Low.
Level of consensus: 89.3% agree; 10.7% neutral; 0% disagree.
Statement 26. In patients with a small annulus, TAVI may be considered but the impact on valve haemodynamics, coronary occlusion and future interventions should be carefully evaluated.
Level of evidence: Low.
Level of consensus: 92.9% agree; 7.1% neutral; 0% disagree.
Statement 27. In patients unsuitable for SAVR and transfemoral TAVI, alternative access TAVI should be considered.
Level of evidence: Low.
Level of consensus: 96.4% agree; 3.6% neutral; 0% disagree.
Statement 28. TAVI is feasible in degenerate bioprosthetic surgical or transcatheter AV.
Level of evidence: Low.
Level of consensus: 92.9% agree; 7.1% neutral; 0% disagree.
Bicuspid AV is a common phenotype among Asian people, with one Chinese study reporting that almost 48% of patients being screened for TAVI had a bicuspid AV.43 The use of TAVI in the treatment of severe AS of bicuspid AVs may be associated with increased procedural complexity and complications.43,44 While guidelines have indicated bicuspid AV as an anatomical factor favouring SAVR, experience of using TAVI to treat bicuspid AS is increasing.4 Registry studies suggest that the outcomes and mid-term prognosis of patients with bicuspid AV treated with TAVI were similar to those with TAVI-treated tricuspid AVs.45 In the larger STS/ ACC Transcatheter Valve Therapies Registry, all-cause mortality at 30 days and 1 year, the rate of moderate or severe paravalvular leak at 30 days and 1 year and improvement in quality of life were similar between groups.46 However, the 30-day stroke rate was significantly higher for bicuspid versus tricuspid AS (2.5% versus 1.6%).47 The risk of procedural complications requiring open heart surgery was also significantly higher in the bicuspid group (0.9% versus 0.4%). Another study showed that, while overt stroke was found in 2.4% versus 1.7%, respectively, of patients with bicuspid and tricuspid AS treated with transcatheter AV replacement (TAVR), 28.6% versus 10.9%, respectively (p=0.005) had brain lesions >1 cm3 on diffusion-weighted MRI.48 Regarding the technical considerations for TAVI in bicuspid AS, meticulous CT planning with established methods of valve sizing, as well as balloon predilation, were recommended. Developments in valve sizing such as supra-annular structure-based sizing strategies for TAVR in patients with bicuspid AS are also emerging.49
A small aortic annulus is another anatomical consideration in the management of severe AS, especially in the Asia-Pacific region. The AsiaPacific TAVI registry found that almost 32% of patients received an AV sized ≤23 mm.1 A few Asian non-randomised studies have reported that TAVI results in favourable haemodynamics in the treatment of patients with severe AS with small annulus.50,51 Importantly, the VIVA trial, a randomised controlled trial that compared TAVI versus SAVR in patients with severe AS with small annulus, found that TAVI delivered similar 2-year outcomes for mortality, stroke and cardiac rehospitalisation events.52 However, these findings should be interpreted with caution as VIVA was underpowered and had a short follow-up. In the recently published SMART trial, for patients with severe AS and small annulus of ≤430 mm2 , a self-expanding supra-annular valve was found to be non-inferior to a balloon-expandable valve with respect to 1 year clinical outcomes and had better valve haemodynamics.53 Patients with small annulus planned for TAVI should be carefully selected and factors such as valve haemodynamics, risk of coronary occlusion and need for future interventions weighed.
Evidence to recommend for or against the use of alternate access TAVI in patients unsuitable for SAVR and transfemoral TAVI is lacking. However, the 2021 ESC guidelines gave a Level IIb recommendation on the use of non-transfemoral TAVI in these patients and the current consensus panel tended to agree with this position. Nevertheless, the consensus panel underscores the importance of shared decisionmaking in these instances because of the lack of hard evidence. In addition, local experience and expertise is crucial on deciding on the optimal route for alternate access TAVI and this may vary from centre to centre.
Lastly, as degeneration of implanted bioprosthetic or transcatheter valves may occur during the patient’s lifetime as shown in Figure 2, subsequent treatment after valve degeneration should be considered in the first instance. Several studies have shown that TAVI is a safe and feasible treatment option in patients with degenerate bioprosthetic AVs.54–56 However, a meta-analysis showed that SAVR was associated with lower all-cause mortality compared with a transcatheter valve-in-valve strategy and may be considered if the patient is at low surgical risk. Similarly, TAVI may also be performed to treat patients with degenerate transcatheter AV.4,57,58 However, the risk of prosthesis–patient mismatch, coronary occlusion and the possibility of future access to the coronary arteries should be carefully evaluated. CT is a crucial tool in the planning of these TAV-in-SAV and TAV-in-TAV procedures. For the smaller surgical bioprosthetic AVs, balloon fracturing where safe may be considered to optimise haemodynamics. This should generally be done post TAVI valve implantation.59,60
Limitations and Conclusion
The statements presented in this paper aim to guide clinicians based on the most updated evidence and collective expert opinion from the AsiaPacific. However, given the varied clinical situations and healthcare resources present in the region, these recommendations should augment clinical judgement rather than replace it. The use of TAVI in the management of AS should be individualised, taking into account the patient’s clinical characteristics as well as patient and caregiver concerns and preferences. Clinicians should also be aware of the challenges that may limit the applicability of these recommendations in their individual centre, such as the access to specific technologies, availability of resources, competency level of clinical staff, accepted local standards of care and cultural factors. Nonetheless, this position statement strives to help create and improve protocols and pathways for the appropriate management of AS and the role of TAVI across the Asia-Pacific region and thus potentially improving patient care and outcomes in AS patients.
Clinical Perspective
- The decision to treat aortic stenosis (AS) should be a shared process informed by multiple considerations, such as the severity of AS, the patient’s symptoms and clinical characteristics, overall surgical risk, available resources and expertise, and considerations on lifetime management.
- The use of TAVI in the management of AS should be individualised after a thorough discussion with the Heart Team and patient, taking into account both the clinical scenario and patient considerations.
- Clinicians should also be aware of the challenges that may limit the applicability of these consensus recommendations in the management of AS in their centres.