Acute decompensated heart failure (ADHF) is a global health burden affecting millions of people across the globe and is associated with high rates of mortality and morbidity.1 Furthermore, ADHF poses a great economic challenge linked to high rehospitalisation rates.2 Heart failure (HF) with preserved ejection fraction (HFpEF) accounts for more than half of total ADHF-related admissions.3 In a general population aged 60 years or older, 4.9% were identified to have HFpEF. This number is expected to increase further with the worsening rate of cardio-metabolic risk factors, including diabetes, hypertension and metabolic syndrome.3,4
Although efforts are increasing to describe the economic consequences linked to ADHF care, there remains sparsity in regional and local data surrounding HFpEF-related ADHF admissions. Large registries on ADHF incorporating data from Malaysian populations include the REPORT- HF and ADHERE-International-Asia Pacific (ADHERE-AP) registries.5,6 Unfortunately, few, if any, efforts have been made by either registry to describe in detail HFpEF-related ADHF admissions. Although a prospective study using the ASIAN-HF registry database has since been published, describing clinical characteristics and outcomes in 1,204 patients with HFpEF, the ASIAN-HF registry comprises purely ambulatory patients with little data on acute admissions.7
A recent prospective cohort study by Tromp et al. using the ASIAN-HF registry, further describes the rising prevalence of multimorbidity among heart failure (HF) patients from 11 regions in Asia.8 Furthermore, the study describes the presence of various multimorbidity groups with varying geographical distribution, through latent class analysis, among the following groups: elderly/AF (old, with more AF); metabolic (obese, diabetic and hypertensive); young (younger, with low prevalence of comorbidities); ischaemic (ischaemic aetiology); and lean diabetic (diabetic, with low prevalence of obesity). HFpEF was commonly associated with the elderly/AF, metabolic and lean diabetic groups, the latter two of which were reportedly common in Malaysia. However, there have been no other studies with reportedly similar findings, which calls into question the accuracy of such multimorbidity grouping. Thus, the aim of our study was to describe the baseline characteristics, clinical parameters and clinical outcomes of patients diagnosed with HFpEF admitted for ADHF in the largest tertiary cardiac centre within Malaysia, with the intention of challenging the current prevailing notion that HFpEF in Asia and southeast Asia are only seen within these multimorbidity phenotypes.5,7,8,9
Methods
Study Centre
A retrospective, observational study was conducted at our institution: Institut Jantung Negara (IJN), Malaysia. IJN is a major cardiac tertiary centre in the heart of Kuala Lumpur and the major referral centre for advanced HF care, including heart transplantation, left ventricular assist device and complex cardiovascular implantable electronic device implantation (CIED). There is also an active inpatient and outpatient HF service providing multidisciplinary care involving cardiologists, cardiothoracic surgeons, pharmacists, nurse specialists, physiotherapists and cardiac rehabilitation specialists.
Study Design and Study Population
Data on ADHF-related hospitalisation and in-hospital mortality from 1 January 2009 to 31 December 2018 were obtained through the IJN-ADHF local registry. Selection was through universal sampling. Patient admission criteria for study inclusions were patients with a history of at least one admission, either de novo or those with a history of previous ADHF admissions, and compulsory recorded left ventricular ejection fraction (LVEF) obtained from echocardiography done either during first admission of ADHF or previously recorded within 3 months prior to the admission. Patients with multiple admissions beyond their first during the study period were only considered for inclusion once, although subsequent readmissions were reviewed to supplement data collection. A total of 4,198 patient admissions were identified. Patients were divided into two separate cohorts: LVEF ≥50% with either evidence of echocardiographic findings to support diastolic dysfunction and/or raised natriuretic peptide levels, and no history of LVEF <50% (i.e. HFpEF); and LVEF <50% (i.e. non-HFpEF).
Patients with acute coronary syndrome (ACS) were not excluded from our study. We believe that ACS remains a common precipitant for decompensation in many HF patients, and to exclude such patients would be misleading to the data presented. The purpose of the present paper is to illustrate the common clinical presentation of HFpEF patients and how they differ from their non-HFpEF counterparts, and universal sampling of those admitted for HF, regardless of precipitant, was pivotal.
Study Variables and Data
Information gathered included baseline demographics, clinical variables, emergency inpatient procedures performed, and clinical outcomes. Baseline demographics included information on age, gender, ethnicity, BMI, presence of comorbidities including coronary artery disease (CAD), history of coronary intervention, history of MI, chronic kidney disease (CKD), AF, diabetes (DM), hypertension, dyslipidaemia, stroke or transient ischaemic attack (TIA), chronic obstructive pulmonary disease (COPD) or asthma, obstructive sleep apnoea (OSA), history of device implantation, smoking status (either current or former), and history of HF hospitalisation (Table 1). Data on aetiology was also obtained. Ischaemic cardiomyopathy was based on patients having been diagnosed with a history of CAD, with evidence of regional wall motion abnormalities corresponding to diseased coronaries but remaining to have LVEF ≥50%.
Clinical variables extracted included vital signs (i.e. systolic blood pressure [SBP] and heart rate [HR]) as well as signs and symptoms on presentation (Table 2). Blood investigations, including levels of haemoglobin, haemoglobin A1C (HbA1c), random blood glucose, total bilirubin, albumin, N-terminal Pro-brain natriuretic peptide (NT-Pro BNP), uric acid, potassium, urea, sodium, and estimated glomerular filtration rate (eGFR) were obtained (Table 2).
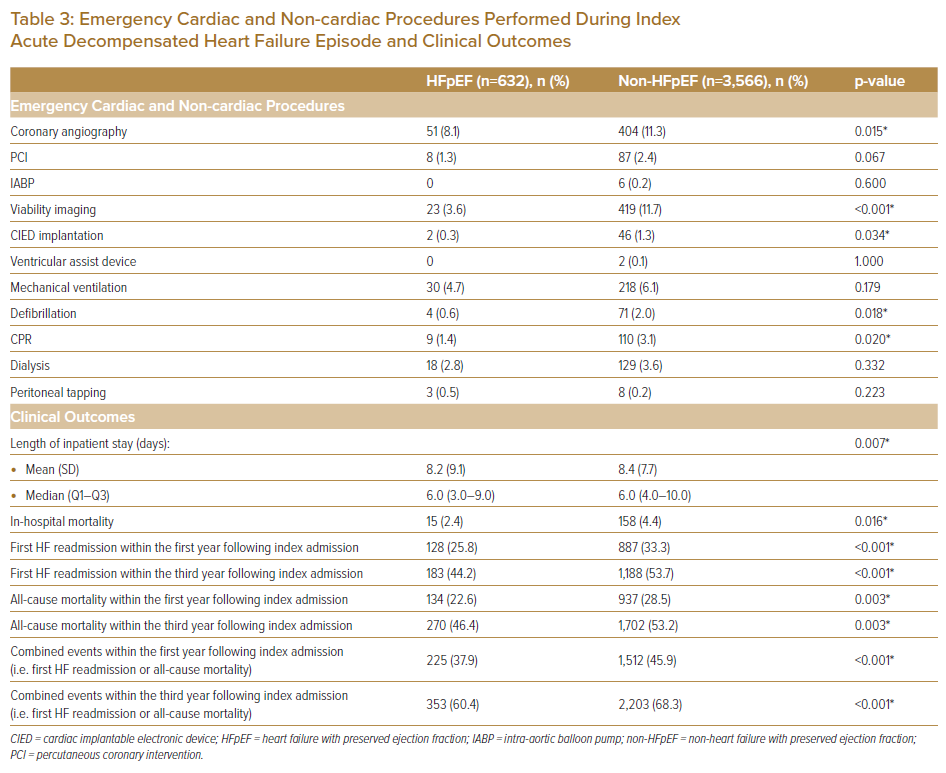
Data on emergency inpatient procedures performed during admissions included cardiac (e.g. coronary angiography [CAG], percutaneous coronary intervention [PCI], intra-aortic balloon pump [IABP], viability imaging, CIED and ventricular assist device [VAD] implantation) and non-cardiac based procedures (e.g. mechanical ventilation, defibrillation, cardiopulmonary resuscitation [CPR], dialysis and peritoneal tapping) (Table 3). Data on clinical outcomes included length of inpatient stay (LOS), in-hospital mortality during the index ADHF event, and events (i.e. first readmission and/or mortality) occurring within the first and third years from index admission (Table 3). Data on medications prescribed on both admission and at discharge were also obtained (Table 4).
Statistical Methods
Descriptive statistics were used to summarise baseline demographic, comorbidities, vital signs, blood investigation, emergency inpatient procedures performed, hospital LOS, and clinical outcomes. Categorical variables were expressed as frequencies and percentages. Continuous variables were summarised as mean (M) with SD or median with interquartile ranges (Q1 and Q3). Categorical variables were analysed to compare between two cohorts, HFpEF and non-HFpEF using the chi-square test or Fisher’s exact test (where applicable), whereas independent t-test or independent-samples Mann–Whitney U-test (where applicable) were carried out to assess the distribution of continuous variables. Kaplan-Meier analysis was used to predict probability of readmission or death at follow-up.
Results
Baseline Demographics, Comorbidities and Aetiology
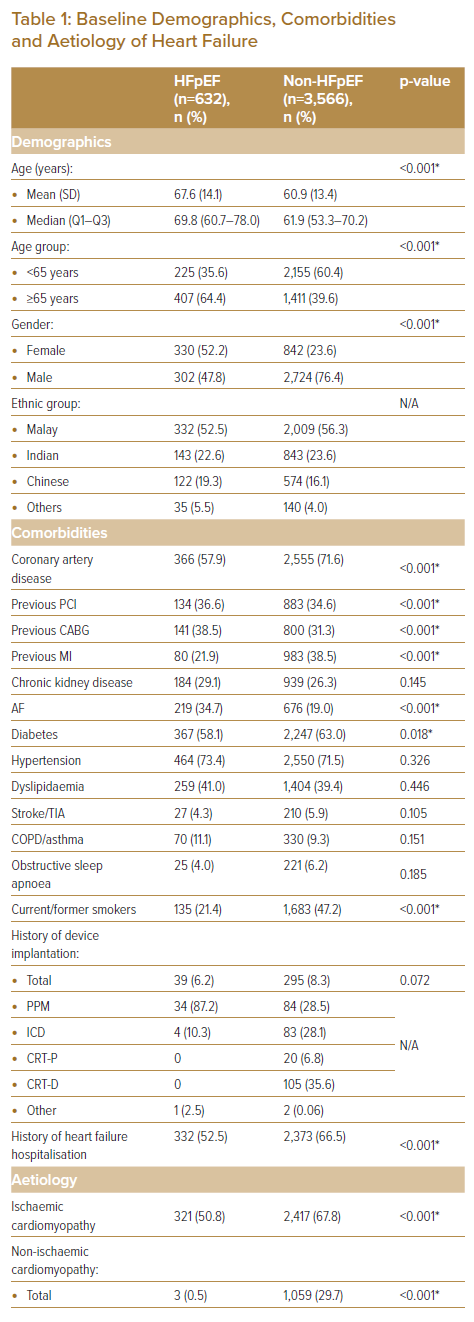
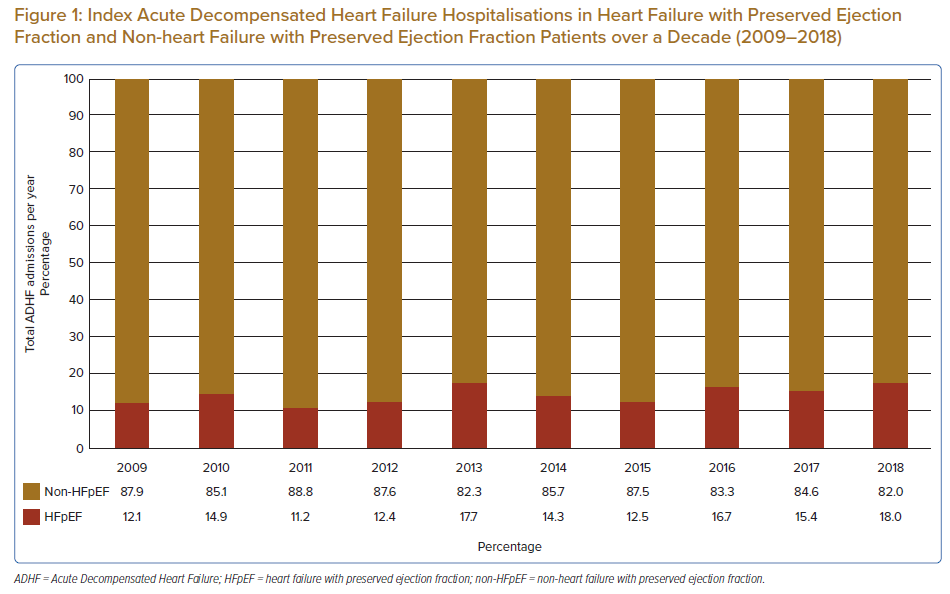
A total of 4,198 patients were identified in the study population, of which 632 (15.1%) were admitted for HFpEF-related ADHF (Table 1). When compared, annual admissions for ADHF mainly comprised of patients who were non-HFpEF, with an equally increasing trend in number of admissions in both cohorts (Figure 1). HFpEF patients were significantly older versus their non-HFpEF counterparts (mean 67.6 + 14.1 versus 60.9 ± 13.4 years respectively, and median 69.8 versus 61.9 years, respectively; p<0.001), and close to two-thirds of HFpEF patients were aged 65 years or older. There were also significantly more females in the HFpEF cohort versus non-HFpEF, with variability in admission rates based on ethnicity.
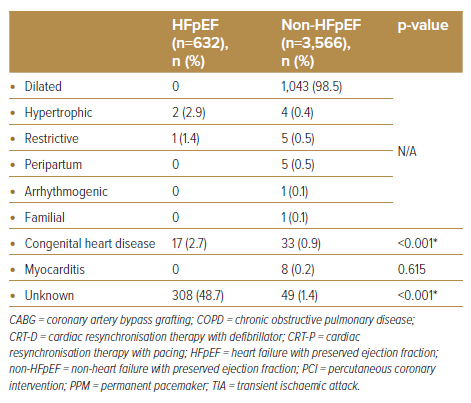
When comparing the HFpEF group to the non-HFpEF cohort, there were far fewer patients with CAD (57.9% versus 71.6%, respectively; p<0.001), history of MI (21.9% versus 38.5% respectively; p<0.001) and diabetes (58.1% versus 63.0%, respectively; p=0.018). AF was also more common among HFpEF patients (34.7% versus 19.0%, respectively; p<0.001). Although ischaemic cardiomyopathy remains a common aetiology among HFpEF patients in our cohort, it is significantly less common when compared to the non-HFpEF group (50.8% versus 67.8%, respectively; p<0.001). A significant number of HFpEF cases was idiopathic in nature versus non-HFpEF patients (48.7% versus 1.4%, respectively; p<0.001).
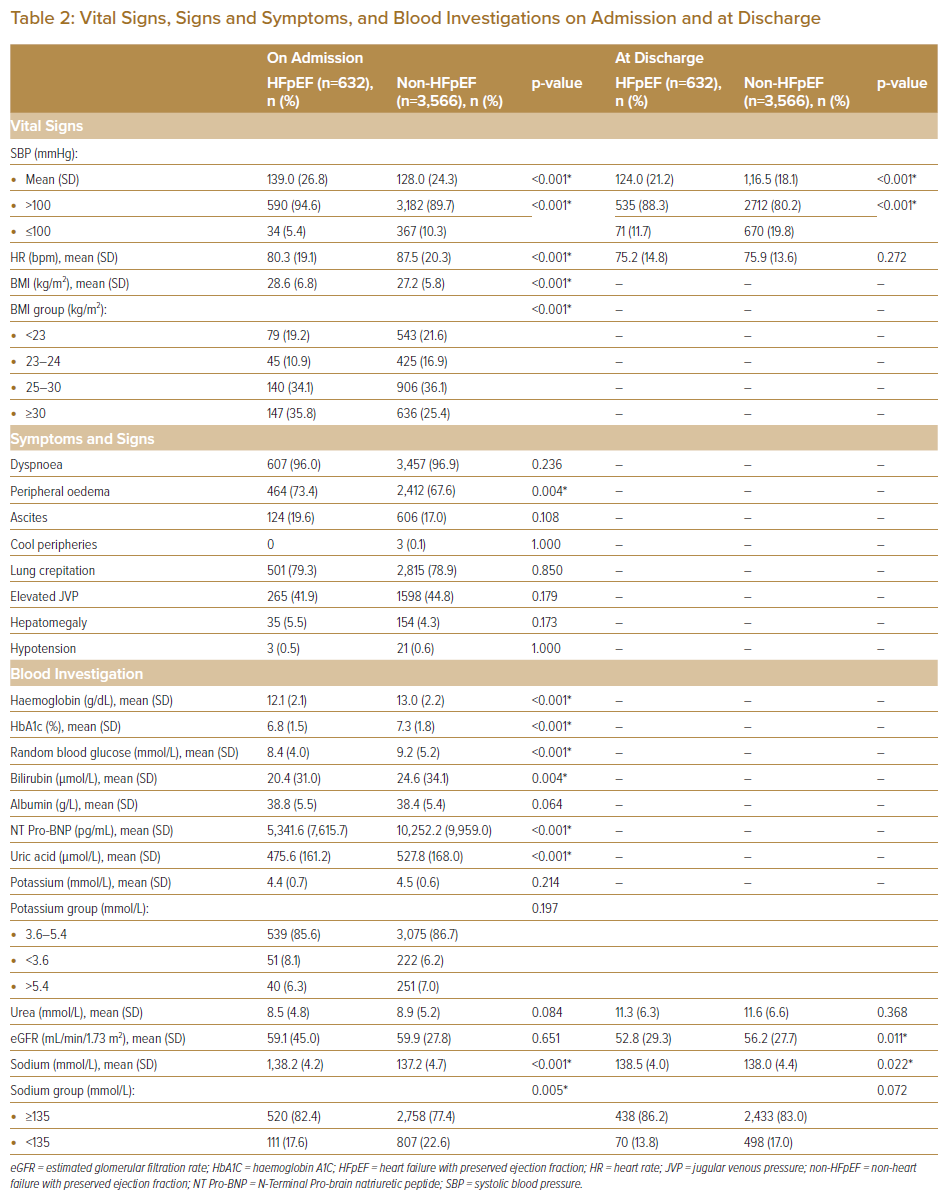
Although known hypertension was no different between cohorts, systolic blood pressure on admission was shown to be significantly higher in the HFpEF cohort versus the non-HFpEF group, possibly suggesting significant under-diagnosis of hypertension in the population. The average BMI was significantly higher in HFpEF patients (28.6 ± 6.8 versus 27.2 ± 5.8 kg/m2, respectively; p<0.001), with more than two-thirds of HFpEF patients having a BMI ≥25 kg/m2 (69.9%). Although dyspnoea (96.0%), peripheral oedema (73.4%) and lung crepitations (79.3%) were common clinical presentations in HFpEF patients, only peripheral oedema was significantly higher when comparing cohorts (73.4% versus 67.6%, respectively; p<0.001).
The majority of blood investigation results significantly differed between both the HFpEF and non-HFpEF groups, with lower haemoglobin levels (12.1 ± 2.1 versus 13. 0 ± 2.2 g/dl, respectively; p<0.001), lower bilirubin (20.4 ± 31.0 versus 24.6 ± 34.1 µmol/l, respectively; p=0.004) and NT-Pro BNP levels (5341.6 ± 7615.7 versus 10252.2 ± 9959.0 pg/ml, respectively; p<0.001), suggestive of possibly less congestion. Unfortunately, there was no reason that could be elucidated from our analysis for the lower rates of haemoglobin levels among HFpEF patients. Although urea (8.5 ± 4.8 versus 8.9 ± 5.2 mmol/l, respectively; p=0.084) and eGFR levels (59.1 ± 45.0 versus 59.9 ± 27.8 ml/min/1.73 m2, respectively; p=0.651) were not significantly different between cohorts at admission, the eGFR values were significantly lower at discharge in the HFpEF cohorts (52.8 ± 29.3 versus 56.2 ± 27.7 ml/min/1.73 m2, respectively; p=0.011).
Of the analysed inpatient cardiac procedures performed, non-HFpEF patients had significantly more CAG, viability imaging and CIED implantation, whereas for non-cardiac procedures, non-HFpEF patients had significantly more defibrillation and CPR. Both the mean duration of hospital LOS was shorter (8.2 ± 9.1 versus 8.4 ± 7.7 days, respectively; p=0.007) and in-hospital mortality was lower (2.4% versus 4.4%, respectively; p=0.016) in HFpEF patients versus non-HFpEF patients.
The number of first HF readmissions within both the first and third years from index admission was lower in the HFpEF cohort. Similarly, the number of all-cause mortalities within both the first and third years from index admission was lower in the HFpEF cohort. The number of composite events (first HF readmissions and all-cause mortality) within both the first and third years from index admission was lower in the HFpEF cohort. When illustrated in a Kaplan-Meier estimate, patients with HFpEF were at lower risk of both composite outcomes and all-cause mortality, up to 10 years from their index hospitalisation (Supplementary Material Figures 1 and 2).
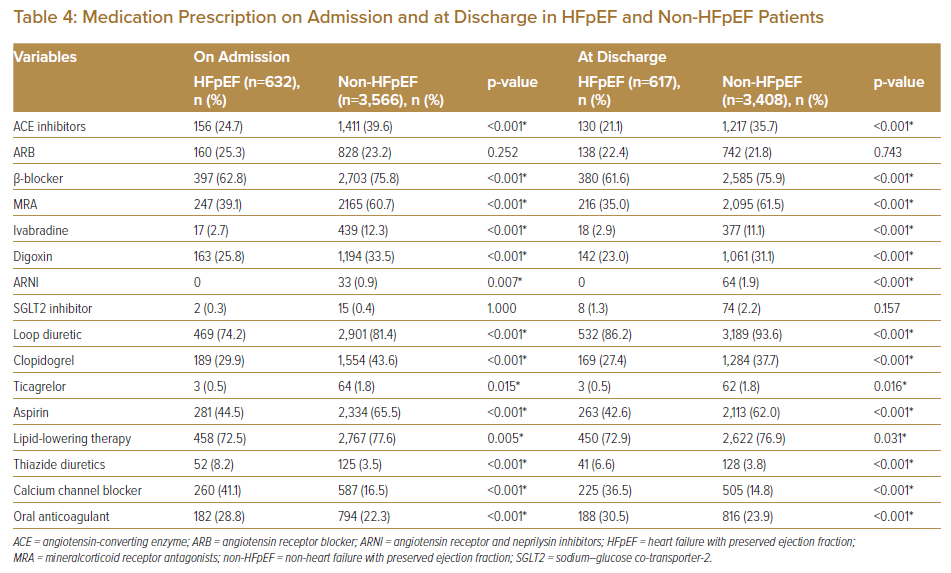
The use of angiotensin-converting enzyme (ACE) inhibitors, beta-blockers and mineralcorticoid receptor antagonists (MRA) were significantly lower in the HFpEF cohort versus the non-HFpEF group. This is consistent with the lack of evidence (or, if present, lower level of recommendation) in guidelines in their use among HFpEF patients. There was also significantly lower use of ivabradine and digoxin in HFpEF patients, both of which are more commonly used in HF with reduced ejection fraction (HFrEF). There was no significant difference in the use of sodium-glucose co-transporter-2 (SGLT2) inhibitors and complete absence in use of angiotensin receptor and neprilysin inhibitors (ARNI) in HFpEF patients, which is expected as evidence for their use in HFpEF has only been recent (the past 3 years). Use of loop diuretics was significantly higher in non-HFpEF patients. Additionally, use of antiplatelet therapies (clopidogrel, ticagrelor and aspirin) and lipid-lowering therapy were also significantly higher in non-HFpEF patients. Both calcium channel blockers and thiazide diuretics (commonly used antihypertensives in Malaysia) were more often prescribed in HFpEF patients. Although the rates of hypertension were not significantly different between cohorts, lower use of conventional antihypertensives in HFrEF patients was not surprising as initiation of guideline-directed medical therapy often led to a lowering of blood pressure significantly, circumventing the need for additional antihypertensives. Use of oral anticoagulation was higher in HFpEF patients, likely due to higher rates of AF in this cohort.
Discussion
Our dataset is unique as it is the first effort, to our knowledge, to describe a HFpEF population within Malaysia. A major source of published data on HFpEF for comparison was derived from the ASIAN-HF registry (both the main registry data and southeast Asian subset), the Asia-Pacific subgroup of the PARAGON-HF trial and a prospective study by Yap et al.7,10,11 Of the three, both the ASIAN-HF and PARAGON-HF studies reported on ambulant HF patients, while Yap et al. reported on HFpEF patients admitted with ADHF. Unfortunately, there are no data published from either INTER-CHF, REPORT-HF or ADHERE-AP specifically describing their HFpEF cohorts, respectively.5,6,12 Nevertheless, an attempt was made to compare the characteristics of HFpEF patients using the accessible registries and studies, to better understand the burden of HFpEF in our region (Supplementary Material Table 1).
The overall prevalence of HFpEF among ADHF patients in our population was low (15.1%). Furthermore, unlike previous reports suggesting that up to 50% of acute hospitalisation for HF is linked to HFpEF patients, a much smaller proportion per annum (12.1–18.0%) exists in our cohort (Figure 1).3 Within a Malaysian context, this has been previously demonstrated as well as among ADHF patients, where 12.7–23.5% of admissions were for HFpEF patients.13–15 The low numbers were likely due to several factors, including severely underdiagnosed HFpEF among patients admitted with dyspnoea, as patients with HFpEF often have co-existing respiratory disease (e.g. COPD, OSA) or CKD, which can presently similarly in the acute setting.7–11 Another reason for low numbers may be the use of an ADHF database for data collection, which is further elaborated under the Limitations section of this paper.
The ethnic distribution of our cohort was similarly consistent with other Malaysian-based registries on ADHF, with no significant difference between HFpEF and non-HFpEF cohorts.13–15 Furthermore, the higher proportion of younger, predominantly Malay or Indian patients parallels the higher proportion of non-HFpEF cases in our overall cohort, all of which are linked to higher rates of ischaemic heart disease, which has been shown to more commonly occur in these ethnicities, often a decade younger than other Western populations.5,8,16–19 This is further supported by higher use of antiplatelets and lipid-lowering therapy among non-HFpEF patients in our study (Table 4).
Our HFpEF cohort consisted of older and female-predominant patients, with significantly higher rates of CAD and the presence of traditional cardiovascular risk factors (e.g. obesity, diabetes and smoking). There was also a higher rate of AF seen in our HFpEF cohort and, as mentioned in an earlier section of this paper, significant rates of hypertension within the HFpEF population, grossly under-appreciated. As illustrated by other studies within the region (Table 4), a similar pattern is seen in terms of average age (67.4–73.1 years), female-preponderance (49.6–64.7%), average BMI values (27.1–28.6 kg/m2) AF (28.5–34.7%) and hypertension (71.0–92.0%).7,10,11 However, although cross-sectional studies in western populations strongly described similar characteristics in HFpEF patients, we now appreciate a greater heterogeneity existing in the condition.20 As highlighted at the beginning of the present article, a cohort study by Tromp et al. identified five different phenotypes of HF (both HFpEF and non-HFpEF combined) within the Asian region.8 Although the HFrEF phenotype, proposed to be common in Malaysia, has been substantiated by various local and regional registries, there have been no data, to date, to support the presence of a similar lean diabetic or metabolic HFpEF phenotype.13–15,21 Our study suggests that if the phenotyping exercise remains true, HFpEF patients in our cohort appear to be distinct from any of the five proposed phenotypes. If forced to choose, our cohort would only slightly resemble a combination of both the metabolic or elderly/AF cohort described, although more detailed analysis is required to assess this (Supplementary Material Table 2).8
There was a relatively higher proportion of HFpEF patients in our cohort who underwent permanent pacemaker (PPM) implantation. This came as no surprise, as a strong correlation between chronotropic incompetence and HFpEF (and consequent exercise intolerance) has been proven widely.22–24 Additionally, a large proportion of HFpEF patients had no known cause to their condition. Unlike non-HFpEF patients, where commonly associated aetiologies like ischaemia are routinely investigated in daily clinical practice (either through CAG or cardiovascular imaging, as seen in our study), the common aetiologies linked to HFpEF are often complex and require a multispeciality (e.g. cardiac amyloidosis or sarcoidosis), multimodality (e.g. infiltrative, storage disease or genetic cardiomyopathies) and multidisciplinary approach.25 Although guidelines have since been developed to aid in the diagnostic pathway of HFpEF (using the HFA-PEFF algorithm), recommendations, such as diastolic stress testing and invasive haemodynamic measurements, are often neither pragmatic nor pursuable by a majority of centres.26 Furthermore, the lack of evidence-based management for HFpEF during the study period may have greatly influenced enthusiasm in obtaining diagnosis beyond that of HFpEF. However, with growing interest in the role of newer pharmacological agents, such as mavacamten for hypertrophic cardiomyopathy and SGLT2 inhibitors across the ejection fraction spectrum of HF, more attention should be paid to obtaining a more precise diagnosis in HFpEF patients.26,27
Only peripheral oedema was significantly more prominent in HFpEF patients versus their non-HFpEF counterparts. Furthermore, our HFpEF cohort appears relatively stable compared to the latter. This is supported by blood investigations suggestive of less congestion (lower total bilirubin and NT-pro BNP levels) on admission, shorter duration of inpatient stay, lower use of CPR and defibrillation, and lower in-hospital mortality rates. Furthermore, the rates of events (either first HF rehospitalisation and all-cause mortality or all-cause mortality on its own) up to 10 years from admission were lower in the HFpEF cohort versus the non-HFpEF group. However, when compared to other registries, specifically the ASIAN-HF cohort, HF rehospitalisation or all-cause mortality within the first year was higher in our cohort (37.9% versus 12.1–23.6%). This is not surprising as rates of all-cause mortality in HF patients in Malaysia and southeast Asia are often among the highest in the region, ranging between 7.2 and 49.7% within the first year.13–15,28 Median NT-Pro BNP levels on admission were also resoundingly higher in our patient population, suggestive of a more severe disease state during hospitalisation, as previously proven.29,30 However, relying on NT-pro BNP levels to determine the severity of congestion in HFpEF has recently been shown to be misleading, and lower or normal levels may, in fact, be associated with more morbid prognosis, highlighting the gap in the utility of NT-pro BNP as a biomarker of diagnosis and prognostication in HFpEF.31
Although it would be interesting to analyse prognostic factors and how they differ between HFpEF and non-HFpEF patients, further analysis on this would detract from the focus of the paper, which was to describe the HFpEF population that exists in our local setting. Furthermore, we understand limitations in analysing data obtained retrospectively, especially over a long duration with possible changes in attitudes and perceptions on diagnosing and managing HFpEF over time. Thus, we have decided against identifying possible prognostic factors in either cohort, although we support any efforts and opportunities for future analysis to be performed in a non-observational database.
Limitations
This study has several limitations. We acknowledge potential bias associated with reporting observational, retrospective data and have taken careful measures to describe association, and not direct causation, throughout the paper. Furthermore, as a single-centred study, it may be difficult to generalise these findings to represent local and regional practice. However, as a centre with data spanning a substantial duration, the analysis provided remains valuable. Another limitation was the use of an ADHF database for data collection. As suggested, our HFpEF cohort was relatively less unwell versus the non-HFpEF cohort, and therefore the possibility of such a group of patients to present in an ambulatory setting, as opposed to through the emergency department, is likely. If so, this may explain the lower proportion of HFpEF patients versus the overall ADHF population.
Using BMI at admission may overestimate the rate of obesity in a HF population, as congestion may increase body weight. Unfortunately, additional analysis using other anthropometric measurements was not performed. Furthermore, there may be doubts about the accuracy of the diagnosis of HFpEF as there is a possibility that LVEF may have initially been lower than required for diagnosis at other time points, 3 months prior to admission, which may lead to misclassification. At present, we are limited by our ability to obtain information retrospectively as IJN is independent of other government or private-based hospitals in Malaysia. Data sharing among healthcare facilities is limited in Malaysia, but we believe that taking the additional step to review echocardiographic data 3 months prior to admission serves as a reasonable effort on our part to limit the possibility of misclassifying HFpEF patients.
Although direct comparison was made to existing HFpEF and HF data published, no statistical calculation was performed to substantiated differences or similarities observed, which can lead to bias opinions. We welcome any collaboration with authors from said publications to improve the data analysis of our local and regional HFpEF population. Our aim was to describe specific data on ADHF patients with HFpEF within Malaysia, which has not been published before. At present, descriptions of HFpEF in most southeast Asian and east Asian countries often reference only a handful of papers, which we believe may not truly be representative of the HFpEF landscape locally. We acknowledge limitations in our analysis and the representativity of the studied population compared with the general population but feel strongly that our granular data is pivotal in understanding how HFpEF patients truly present in the acute setting, with significant differences in clinical presentation compared to other Asian HFpEF patients.
Conclusion
Our study highlights key background information and clinical parameters of HFpEF patients admitted for ADHF, a first of its kind in Malaysia and one of the few efforts from the southeast Asian region. Our study highlights the unique phenotype of HFpEF patients in Malaysia, sharing many similarities with other previous regional reports but showcasing key differences in clinical outcomes, including higher 1-year mortality rates. Furthermore, our study challenges the notion of the five major phenotypes of HF previously proposed by the ASIAN-HF group, suggesting instead a unique Malaysian cohort which would not otherwise fit such pigeonholing. It, therefore, highlights how granularity in data collection and analysis is key, especially in such a heterogenous condition as HFpEF.
Clinical Perspective
- Heart failure with preserved ejection fraction (HFpEF) remains an elusive and poorly managed condition in Asia.
- This study provides a much-needed insight into the difference in acute clinical presentation between HFpEF and their reduced ejection fraction counterparts.
- This study also challenges the notion of the major phenotypes of heart failure proposed by previous studies, highlighting the importance of granularity in data, especially in a heterogenous condition like HFpEF.