Transcatheter electrosurgery refers to the novel technique of traversing or cutting tissue, within blood-filled spaces, using alternating current directed by guidewires and catheters.1 Unlike conventional open surgery, which employs electrosurgery using an electrosurgery pencil in dry, air-filled conditions under direct operator vision, transcatheter electrosurgery uses a long guidewire with insulating microcatheters to concentrate energy to the target tissue within blood medium under fluoroscopy under echocardiographic guidance. This technique is increasingly used in the field of structural heart interventions. Here we report the first series of three different techniques of transcatheter electrosurgery in Asia.
Case 1: Iatrogenic Caval−Aortic Access
An 82-year-old man with severe symptomatic aortic stenosis was deemed surgically inoperable because of multiple medical comorbidities. The multidisciplinary heart team recommended transcatheter aortic valve implantation (TAVI); however, he had severe peripheral vascular disease, making standard transfemoral access impossible.
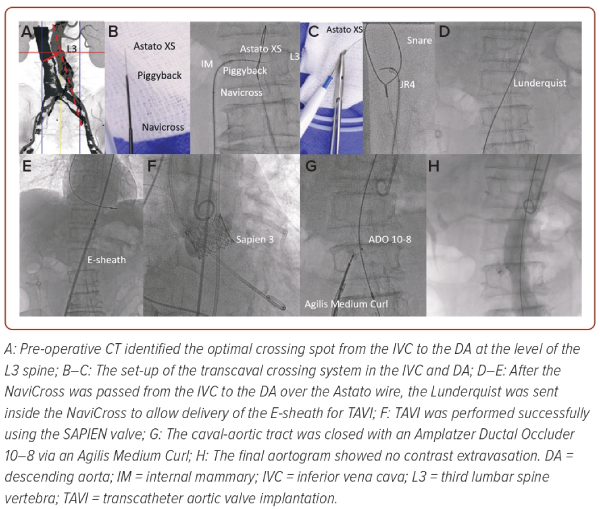
Transcaval access (i.e. an iatrogenic channel from the inferior vena cava [IVC] to the descending aorta) was contemplated with the optimal crossing spot from IVC to the descending aorta identified by CT at the top of the third lumbar spine vertebra (L3; Figure 1A).2 During the procedure, a 300 cm Astato XS 20 (Asahi Intecc) inside a Piggyback wire converter (Teleflex), a 0.035" NaviCross microcatheter (Terumo) and an internal mammary guide catheter were inserted into the IVC and directed to the descending aorta (Figure 1B). In the descending aorta, a 25 mm gooseneck snare inside a JR guide catheter was positioned at the top of the L3 to act as the ‘target’ (Figure 1C).
Under biplane fluoroscopic guidance the Astato, tightly clamped to a diathermy and fired at 70 watts cutting mode for 1 second, was pushed from the IVC to descending aorta (Figure 1C). The Astato wire was then snared by the gooseneck snare into the JR guide and brought up to the proximal descending aorta. Over the Astato wire, the Piggyback and the NaviCross were delivered from the IVC to the descending aorta. The Astato wire and Piggyback were then removed and a Lunderquist wire was delivered inside the NaviCross to the descending aorta (Figure 1D).
Afterwards, the 14 Fr E-sheath (Edwards) was inserted over the Lunderquist wire (Cook Medical) from the IVC to the proximal descending aorta (Figure 1E). Successful TAVI was performed using a SAPIEN 3 valve (Edwards; Figure 1F). A 10–8 mm Amplatzer Ductal Occluder (Abbott) was used to close the iatrogenic tract between the descending aorta and IVC (Figure 1G). The final aortogram showed complete sealing without active contrast extravasation (Figure 1H).
Case 2: Left Coronary Leaflet Laceration
A 75-year-old woman with diabetes, hypertension and AF was admitted for heart failure. She was diagnosed with severe aortic stenosis, but her frailty made her surgically inoperable. The heart team recommended TAVI. Pre-operative CT found a low left main height (6.9 mm) and a narrow left sinus of Valsalva (24.7 mm), posing a significant risk of coronary occlusion after TAVI (Figure 2A). A technique of intentional laceration of the aortic leaflet to prevent iatrogenic coronary artery obstruction during TAVI was chosen to mitigate the risk of coronary occlusion.3
A multipurpose guide catheter with a gooseneck snare was placed in the left ventricular outflow tract (LVOT), then an AL-2 guide catheter with an Astato wire inside a Piggyback was positioned towards the LVOT in front of the left main takeoff under fluoroscopic and transoesophageal echocardiogram (TOE) guidance. The Astato was electrified as above at 70 watts and pushed 3 mm to transverse the left coronary leaflet (LCL; Figure 2C). The Astato wire was snared and externalised outside the body through the MPA guide catheter (Figure 2D).
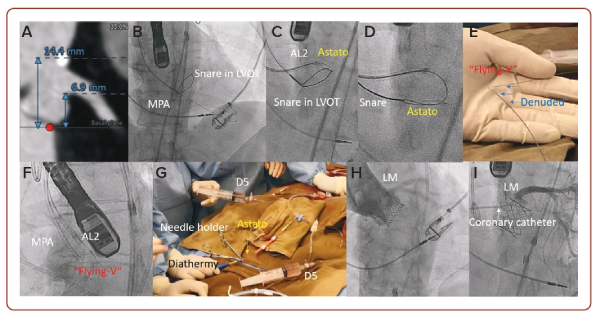
A ‘flying-V’ kink was made on the body of the Astato wire with its inner coating scratched off to improve conductivity (Figure 2E). The ‘flying V’ was then fed into the body until it straddled against the LCL. The two guide catheters were then pulled simultaneously, with the Astato wire electrified at 70 watts and the guide catheter flushed continuously with 5% dextrose solution to insulate the system and concentrate energy to the denuded ‘flying-V’ to lacerate the LCL (Figure 2G). After lacerating the LCL, a SAPIEN 3 valve was implanted to treat the patient’s aortic stenosis, while maintaining the coronary perfusion via the split LCL (Figures 2H and 2I).
Case 3: Anterior Mitral Leaflet Laceration
A 65-year-old man with multiple medical conditions and had a prior tissue aortic valve replacement, mitral valve repair using a Memo 3D ring (32 mm, Sorin), tricuspid valve repair and left atrial appendage clip. He had class III heart failure because of a recurrent severe mitral regurgitation (Figure 3A). The heart team recommended valve-in-ring transcatheter mitral valve replacement (TMVR). In view of the presence of a long anterior mitral valve leaflet (AMVL), which posed risk of LVOT obstruction after TMVR, we elected to create an arterial-venous rail upfront in preparation for a tip-to-base laceration of the AMVL if needed (Figure 3B).4
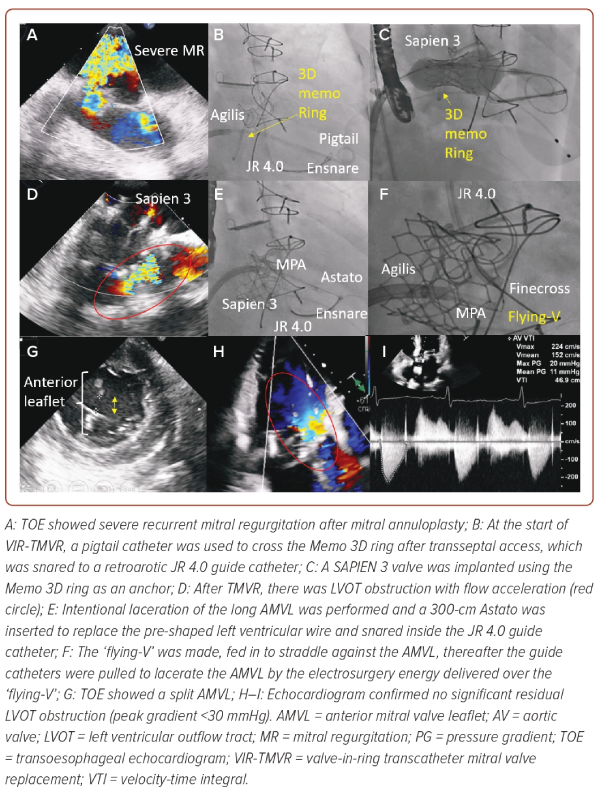
After transseptal access, we crossed the mitral valve using a pigtail catheter, which was snared to a retro-aortic JR4 7 Fr guide catheter. The pigtail was then exchanged to a pre-shaped Safari wire (Boston Scientific) for valve delivery. A SAPIEN valve was deployed successfully anchoring on the Memo 3D ring. However, a LVOT obstruction was noted because of the overhanging long AMVL (Figure 3D). The valve delivery system was replaced with an Agilis Medium Curl Catheter (Terumo) over the Safari wire. The Safari wire was then exchanged to an Astato wire over an MPA guide catheter, snared and externalised through the retro-aortic JR4 guide catheter. A ‘flying-V’ was created as above and fed in to straddle against the AMVL. Under TOE, the ‘flying-V’ was positioned at the A2 scallop. The two guide catheters were pulled simultaneously to lacerate the AMVL with the Astato wire electrified at 70 watts (Figure 3F). A split in the AMVL was confirmed by TOE and resolved the LVOT obstruction (Figure 3G–I).
Discussion
TAVI and TMVR have shown promising data and are increasingly adopted in Asia. Transcatheter electrosurgery has been developed to facilitate valve delivery and mitigate potential complications of TAVI and TMVR. Although technically challenging, these procedures are potentially valuable in Asian patients because of their smaller body build (e.g. a smaller sinus of Valsalva posing higher risk of coronary occlusion, smaller vascular access requiring alternative access for TAVI and smaller left ventricular cavity posing a higher risk of LVOT obstruction). Furthermore, improvements in techniques and equipment have made transcatheter electrosurgery more streamlined, easier and safer to perform.1 It has also been further expanded to treat other cardiovascular diseases and will potentially further revolutionise the field of cardiovascular interventions.1 There is a need to adopt transcatheter electrosurgery in Asia to treat more surgically inoperable patients, including proper simulation, bench training and clinical proctorship. 
Clinical Perspective
- Transcatheter electrosurgery refers to the novel technique to traverse or cut tissue within blood-filled spaces using alternating current directed by guidewires and catheters.
- Although technically challenging, these procedures are potentially valuable in Asian patients because of their small body build posing a higher risk of coronary or left ventricular outflow tract obstruction during transcatheter valve implantation.
- There is a need to adopt transcatheter electrosurgery in Asia to treat more surgically inoperable patients, including proper simulation, bench training and clinical proctorship.