In the Asia-Pacific region, heart failure (HF) is associated with significant health and socioeconomic burdens. Despite registry and epidemiological data indicating that HF patients in the Asia-Pacific are younger and have more severe signs and symptoms compared with their counterparts in Western countries, most local guidelines draw their evidence from clinical studies where patients from the Asia-Pacific region are under-represented.1 Furthermore, regional differences in treatment practices are likely to affect patient outcomes. With this in mind, there is a need for a unified but simple and practical approach to managing chronic HF in the region. Hence, the Asian Pacific Society of Cardiology (APSC) developed these consensus recommendations for the diagnosis and treatment of HF to guide general cardiologists and internal medicine specialists practising cardiology.
Methods
The APSC convened an expert consensus panel to review the literature on the diagnosis and management of chronic HF, discuss gaps in current management, determine areas where further guidance is needed and develop consensus recommendations on the diagnosis, assessment and treatment of chronic HF. The 19 experts on the panel are members of the APSC who were nominated by national societies and endorsed by the APSC consensus board or invited international experts.
After a comprehensive literature search, selected articles were reviewed and appraised using the Grading of Recommendations Assessment, Development, and Evaluation system, with the level of evidence as follows:
- High (authors have high confidence that the true effect is similar to the estimated effect.
- Moderate (authors believe that the true effect is probably close to the estimated effect).
- Low (true effect might be markedly different from the estimated effect).
- Very low (true effect is probably markedly different from the estimated effect).13
As indicated in these levels, the authors adjusted the level of evidence if the estimated effect may differ from the published evidence due to various factors, such as ethnicity, cultural differences and/or healthcare systems and resources, when applied in the Asia-Pacific region.
The available evidence was then discussed during a virtual consensus meeting in April 2022. During the meeting, the panel agreed to limit the consensus statements to the diagnosis and assessment of HF; pharmacotherapy of HF with reduced, mildly reduced and preserved ejection fraction (HFrEF, HFmrEF and HFpEF) as defined by the European Society of Cardiology; and cardiac rehabilitation and referral for cardiac implantable electronic devices (CIED).3 The management of acute HF and decompensated HF, the role of mechanical circulatory support and heart transplant and the treatment of cardiac and non-cardiac comorbidities were beyond the scope of this document and some of these topics will be discussed in future publications.
Consensus recommendations were developed during the meeting. The final consensus statements were then put to an online vote, with each recommendation being voted on by each panel member using a three-point scale – agree, neutral, or disagree. Consensus was reached when 80% of votes for a recommendation were agree or neutral. In the case of non-consensus, the recommendations were discussed further by email and revised accordingly until the criteria for consensus were fulfilled.
Consensus Recommendations
Diagnosis
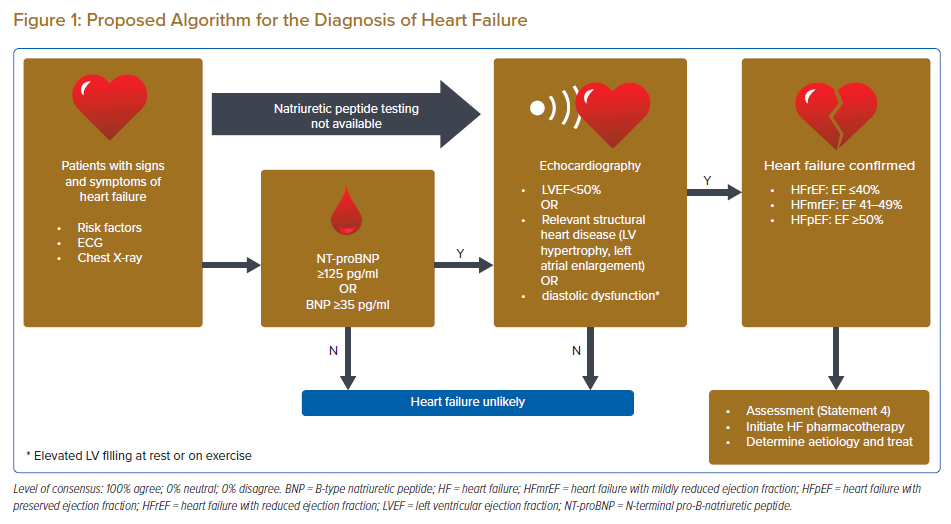
Statement 1. After a careful history and physical examination, the following tests are recommended in patients suspected to have HF: ECG, N-terminal pro-B-natriuretic peptide (NT-proBNP) (preferred) or B-type natriuretic peptide (BNP), transthoracic echocardiograms (TTE) and chest X-ray.
Level of evidence: Low.
Level of consensus: 94.7% agree; 5.3% neutral; 0% disagree.
Statement 2. A plasma concentration of NT-proBNP <125 pg/ml or BNP <35 pg/ml makes a diagnosis of HF unlikely.
Level of evidence: Moderate.
Level of consensus: 100% agree; 0% neutral; 0% disagree.
Statement 3. If BNP/NT-proBNP testing is not available, TTE may be used to diagnose HF.
Level of evidence: Low.
Level of consensus: 100% agree; 0% neutral; 0% disagree.
The panellists agreed that the diagnosis of chronic HF is primarily clinical and rests on the presence of signs and symptoms of HF, especially in patients with risk factors for HF, such as a history of MI, arterial hypertension, coronary artery disease, diabetes, alcohol misuse, chronic kidney disease, cardiotoxic chemotherapy, and in those with a family history of cardiomyopathy or sudden death. However, symptoms and signs alone lack the accuracy required to diagnose HF.4–7 Hence, the diagnosis should be supported by objective evidence of cardiac dysfunction. The panel recommends the following diagnostic tests in patients with suspected chronic HF: ECG, NT-proBNP (preferred) or BNP, TTE, and a chest X-ray.
An ECG is recommended because a normal ECG makes the diagnosis of HF unlikely.4 On the other hand, the ECG may reveal abnormalities such as AF, Q waves, left ventricular (LV) hypertrophy (LVH) and a widened QRS complex, which all increase the likelihood of a diagnosis of HF and may also guide therapy. A chest X-ray is another accessible diagnostic that should be routinely performed as it can provide objective supporting evidence such as cardiomegaly or pulmonary congestion.
The measurement of natriuretic peptides (NP) is recommended, if available, as a plasma concentration of NT-proBNP <125 pg/ml or BNP <35 pg/ml makes a diagnosis of HF unlikely.8,9 The panellists indicated a preference for NT-proBNP due to the following limitations of BNP: it is stable in whole blood for 24 hours at room temperature with the addition of ethylenediamine tetraacetic acid, whereas NT-proBNP is stable for at least 72 hours in whole blood at room temperature requiring no additives; BNP will be increased in patients taking an angiotensin receptor–neprilysin inhibitor (ARNI), which may affect diagnosis.10 Clinicians should also be mindful of conditions that may affect NT-proBNP or BNP. These conditions include AF, increasing age and kidney disease (which may increase NPs) or obesity (which may lower NPs).11,12
However, most of the panellists agreed that NP testing may not be available in many local hospitals in the Asia-Pacific region. Data from the Indian College of Cardiology National Heart Failure Registry found that only 29% of HF patients had biomarker data and this limited usage was attributed to socioeconomic factors.13 Data from other Asia-Pacific countries is lacking. Hence, in areas where NP testing is not feasible, the diagnosis would need to be supported objectively by echocardiography. This modality provides useful information of cardiac function, such as left ventricular ejection fraction (LVEF), chamber size, eccentric or concentric LVH, regional wall motion abnormalities, right ventricular function, pulmonary hypertension, valvular function and markers of diastolic function.14,15
Finally, the experts agreed that the diagnosis of HF may be challenging in some types of patients, such as older women or those with obesity or chronic obstructive pulmonary disease. Furthermore, some panellists emphasised that the aforementioned NP cutoffs may not be able to exclude the possibility of HFpEF in some patients. Hence, if the clinician highly suspects HF in patients with normal NP and echocardiogram results, a referral to an HF specialist is warranted.
The diagnosis of HF is summarised in Figure 1. Regarding this figure, the objective evidence of diastolic dysfunction or raised LV filling pressure parameters will increase the diagnostic accuracy for HFpEF.
Assessment
Statement 4. The following tests should be performed in patients diagnosed with HF: electrolytes and renal function tests, fasting glucose and HbA1c, iron status – transferrin saturation (TSAT) and ferritin – liver and thyroid function tests, lipids and full blood count.
Level of evidence: Low.
Level of consensus: 100% agree; 0% neutral; 0% disagree.
Basic investigations such as electrolytes and renal function tests, liver and thyroid function tests, fasting glucose and HbA1c, lipids, iron status (TSAT and ferritin) and full blood count are recommended to differentiate HF from other conditions and to identify other conditions that may affect the clinical course, prognosis or management of the patient with HF. Other panellists also recommended measuring the international normalised ratio as part of the initial assessment.
Pharmacological Treatment of HFrEF
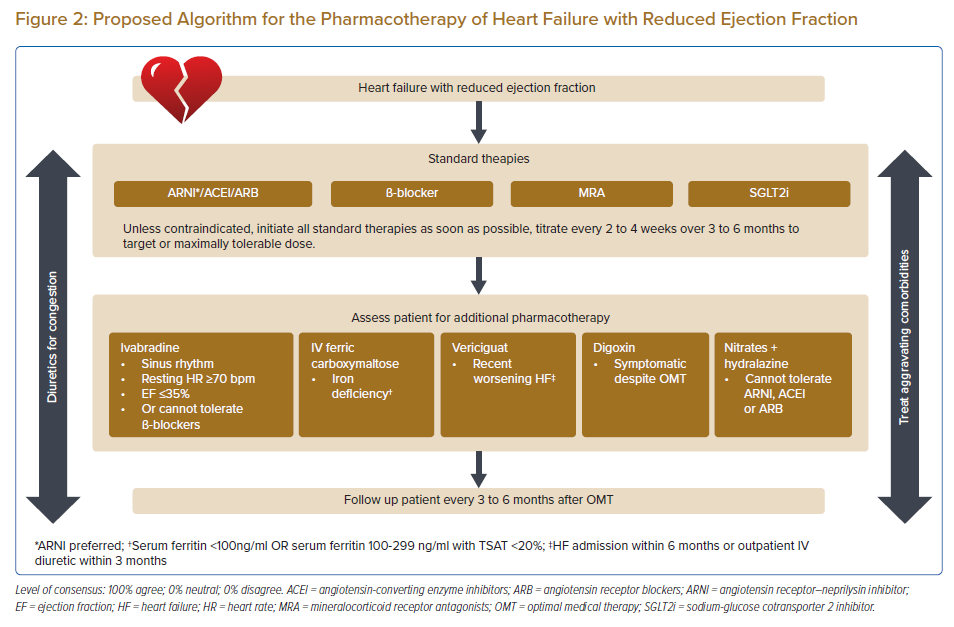
Statement 5. Unless contraindicated, an ARNI (preferred)/ angiotensin-converting enzyme inhibitors (ACEI)/angiotensin receptor blockers (ARB), a β-blocker, a mineralocorticoid receptor antagonists (MRA) and a sodium-glucose cotransporter 2 inhibitor (SGLT2i) are recommended for all HFrEF patients. Initiate these standard therapies as soon as possible.
Level of evidence: Moderate.
Level of consensus: 100% agree; 0% neutral; 0% disagree.
Statement 6. Titration of these standard therapies every 2 to 4 weeks to target or maximally tolerable dose within 3 to 6 months is recommended.
Level of evidence: Low.
Level of consensus: 94.7% agree; 5.3% neutral; 0% disagree.
Statement 7. Loop diuretics are recommended in HFrEF patients with signs and/or symptoms of congestion.
Level of evidence: Low.
Level of consensus: 100% agree; 0% neutral; 0% disagree.
Statement 8. Ivabradine should be considered in HFrEF patients who have EF ≤35% in sinus rhythm and a resting heart rate ≥70 bpm and remain symptomatic despite optimal medical therapy (OMT) including a maximally tolerated ß-blocker. Ivabradine should also be considered for those who cannot tolerate ß-blockers.
Level of evidence: Moderate.
Level of consensus: 100% agree; 0% neutral; 0% disagree.
Statement 9. IV ferric carboxymaltose should be considered in symptomatic HFrEF patients with iron deficiency (serum ferritin <100 ng/ml or serum ferritin 100–299 ng/ml with TSAT <20%).
Level of evidence: Moderate.
Level of consensus: 100% agree; 0% neutral; 0% disagree.
Statement 10. Vericiguat may be considered in HFrEF patients who have worsening HF – HF-related hospital admission within 6 months or outpatient IV diuretic within 3 months – despite OMT.
Level of evidence: Moderate.
Level of consensus: 100% agree; 0% neutral; 0% disagree.
Statement 11. Digoxin may be considered in HFrEF patients who remain symptomatic despite OMT.
Level of evidence: Low.
Level of consensus: 89.5% agree; 10.5%; neutral; 0% disagree.
Statement 12. Nitrate plus hydralazine may be considered for HFrEF patients who cannot tolerate ACEI, ARB or ARNI.
Level of evidence: Low.
Level of consensus: 94.7% agree; 5.3% neutral; 0% disagree.
The panellists unanimously agreed that unless contraindicated, all patients with HFrEF should receive a renin-angiotensin system (RAS) blocker, a β-blocker, an MRA and an SGLT2i. The triad of a RAS blocker (ARNI/ACEI), a β-blocker and an MRA is well established in the treatment of HFrEF to improve survival, lower the risk of HF hospitalisations and reduce symptoms.16–18 The panellists have indicated a preference for ARNIs over ACEIs in patients with HFrEF based on the findings of several well-designed randomised controlled trials (RCTs).19–23 However, if patients cannot tolerate ARNIs or ACEIs, ARBs may be used instead. It should be noted that only a few ARBs (candesartan, losartan and valsartan) are supported by RCTs and no ARB has yet been shown to reduce all-cause mortality.24–27 However, ARBs may reduce the chronic inflammation that characterises and adversely affects the clinical outcomes of patients with chronic HF.28
The SGLT2is dapagliflozin and empagliflozin have been shown to reduce the risk of cardiovascular (CV) death and worsening HF in patients with HFrEF when added to the classical triad of RAS blocker, β-blocker and MRA.18,29 Hence, the panellists agreed to recommend this drug class as part of the foundational treatment of HFrEF in patients with or without diabetes.
It is important that these four foundational treatments for HFrEF should be uptitrated to the target or maximally tolerated dose as soon as possible. Ideally, titration should be done every 2 weeks but may be increased to every 4 weeks, depending on the healthcare resources available in the local setting. Likewise, the target or maximally tolerable dose should ideally be achieved within 3 months but may be extended up to 6 months if healthcare resources are restrictive. Note that the maximally tolerated dose may be slightly lower in Asian people compared with Western patients due to differences in body size. Uptitration of an individual medication should not be done at the expense of the addition of one of the other four foundational medications.
Loop diuretics are recommended to induce euvolaemia in patients with congestion. While the quality of evidence for diuretics is poor, most large-scale landmark trials allowed the use of diuretics when indicated. Pooled analyses also showed that diuretics may reduce the risk of death and worsening HF and may also improve exercise capacity in patients with HFrEF.30 The lowest dose to achieve euvolaemia should be used. The preference for loop diuretics is based on various factors, including greater diuresis without dramatically altering electrolyte levels, broader therapeutic window and drug availability in the region.
The panellists recommended that ivabradine should be considered in HFrEF patients with EF ≤35% in sinus rhythm and a resting heart rate ≥70 bpm who remain symptomatic despite OMT (including maximally tolerated β-blocker) or for those who cannot tolerate β-blocker. Ivabradine is effective only in patients in sinus rhythm and acts by reducing the heart rate. Ivabradine reduced the combined endpoint of CV mortality and HF hospitalisation in the aforementioned subset of patients who were already on an ACEI/ARB, a β-blocker and an MRA.31,32 However, given that the clinical evidence for ivabradine lies mostly in patients on maximally tolerated β-blocker, every effort should be taken to uptitrate the dose of β-blocker. However, the SHIFT trial found that even patients who did not receive any β-blocker due to intolerance had a significant reduction in the risk of the primary endpoint (p=0.012), suggesting that ivabradine may be used as an alternative to β-blocker in this patient subset.32
The panellists voted that IV ferric carboxymaltose should be considered in symptomatic HFrEF patients with iron deficiency (serum ferritin <100 ng/ml or serum ferritin 100–299 ng/ml with TSAT <20%). According to CONFIRM-HF, a multicentre, double-blind, placebo-controlled trial that enrolled 304 ambulatory symptomatic HF patients with LVEF ≤45%, elevated natriuretic peptides and iron deficiency, ferric carboxymaltose significantly prolonged 6-minute walk test distance at week 24 (p=0.002) and this benefit was sustained to week 52 (p<0.001). Treatment was also associated with a lower risk of hospitalisation for heart failure (p=0.009).33 There was insufficient data to make a recommendation for other forms of IV iron and oral iron therapy is not effective in replenishing iron stores and improving exercise capacity in patients with HFrEF and iron deficiency.34
The panel voted to consider vericiguat in HFrEF patients who have worsening HF – admission for HF within 6 months or outpatient IV diuretic within 3 months – despite OMT. This was based on the Victoria trial, which found that in patients with HFrEF and recently decompensated chronic HF, vericiguat reduced the risk of death from CV causes or hospitalisation for HF.35
The panel voted that digoxin may be considered in HFrEF patients who remain symptomatic despite OMT. The Digitalis Investigation Group (DIG) study found that digoxin may be considered in patients with HFrEF in sinus rhythm to reduce the risk of hospitalisation.36 However, the benefit of digoxin in HFrEF patients with AF remains unclear due to a lack of RCT data. Some meta-analyses, which primarily included observational data, have suggested a potentially higher risk of harm in HF patients with AF receiving digoxin, whereas another meta-analysis of non-RCT data found no adverse effects on mortality.37–39 Given the lack of clear evidence and the narrow therapeutic window of digoxin, the use of this drug in HFrEF with AF may be limited to those with a rapid ventricular rate when other therapeutic options are ineffective or contraindicated.38,40,41 The risk of digoxin toxicity is also increased in patients with renal failure; hence, digoxin should be used with caution and may require dose reduction.
Lastly, the combination of a nitrate plus hydralazine may be considered for HFrEF patients who cannot tolerate ACEI, ARB or ARNI. However, despite the high level of consensus, it should be noted that this recommendation is based on an old RCT that included only 642 men with symptomatic HFrEF who were already on digoxin and diuretic treatment, with prazosin as the comparator.42
The pharmacological therapy of HFrEF is summarised in Figure 2.
Pharmacological Treatment of HFmrEF
Statement 13. Loop diuretics are recommended in HFmrEF patients with signs and/or symptoms of congestion.
Level of evidence: Low.
Level of consensus: 100% agree; 0% neutral; 0% disagree.
Statement 14. An evidence-based SGLT2i should be considered in HFmrEF patients to lower the risk of HF hospitalisation or CV death.
Level of evidence: Moderate
Level of consensus: 100% agree; 0% neutral; 0% disagree.
Statement 15. ARNI/ACEI/ARB, β-blocker and MRA may be considered for HFmrEF patients to lower the risk of HF hospitalisation or death.
Level of evidence: Low.
Level of consensus: 100% agree; 0% neutral; 0% disagree.
Statement 16. IV ferric carboxymaltose may be considered in symptomatic HFmrEF patients and iron deficiency (serum ferritin <100 ng/ml or serum ferritin 100–299 ng/ml with TSAT <20%).
Level of evidence: Low.
Level of consensus: 100% agree; 0% neutral; 0% disagree.
As in other forms of HF, diuretics should be used to control signs and symptoms of congestion.
SGLT2is are being actively investigated for their benefit in patients with HFmrEF and HFpEF. The EMPEROR-Preserved trial showed that in patients with symptomatic HF with LVEF >40% and elevated natriuretic peptides, empagliflozin prolonged the time to HF hospitalisation or CV death, with the benefit being mostly driven by a delay in time to HF hospitalisation.43 The clinical benefit of empagliflozin was observed in patients with and without diabetes at baseline. Furthermore, subgroup analysis showed that there was no significant interaction between the primary outcome and the EF subgroup (41–49, 50–<60, and >60%). Additionally, the DELIVER study showed that dapagliflozin was able to significantly reduce the primary composite outcome of worsening HF or cardiovascular death (p<0.001).44 Similar to EMPEROR-Preserved, subgroup analyses of DELIVER showed that the benefit of dapagliflozin was consistent across EF subgroups (41–49, 50–<60, and >60%). These findings indicate that the two SGLTis should be considered for patients with HFmrEF.
While there are no specific trials that included only HFmrEF patients, subgroup analyses of studies that included HFmrEF patients have suggested that the use of the classical triad of an ARNI/ACEI/ARN, a β-blocker and an MRA for patients with an LVEF of 41–49% may reduce the risk of HF hospitalisation or death.45–49
In the CONFIRM-HF, 53% of included patients had an LVEF of 41 to 45% and subanalysis showed that LVEF had no significant interaction with outcomes (p=0.086).33 This suggests that some patients with HFmrEF and iron deficiency, particularly those with LVEF in the lower ranges, would likely benefit from ferric carboxymaltose therapy.
Pharmacological Treatment of HFpEF
Statement 17. Loop diuretics are recommended in HFpEF patients with signs and/or symptoms of congestion.
Level of evidence: Low.
Level of consensus: 100% agree; 0% neutral; 0% disagree.
Statement 18. An evidence-based SGLT2i should be considered in HFpEF patients to lower the risk of HF hospitalisation or CV death.
Level of evidence: High.
Level of consensus: 100% agree; 0% neutral; 0% disagree.
Statement 19. An MRA may be considered for HFpEF patients to reduce the risk of HF hospitalisation.
Level of evidence: Low.
Level of consensus: 89.5% agree; 10.5%; neutral; 0% disagree.
Statement 20. An ARNI or ARB may be considered for patients with HFpEF to reduce the risk of HF hospitalisation or CV death. An ARNI or ARB may be considered for patients with HFpEF to reduce the risk of HF hospitalisation or CV death.
Level of evidence: Low.
Level of consensus: 84.2% agree; 5.3% neutral; 0% disagree.
As in other forms of HF, diuretics should be used to control signs and symptoms of congestion.
In the EMPEROR-Preserved trial, which demonstrated the clinical benefit of empagliflozin in symptomatic HF patients with LVEF >40%, the primary endpoint did not have significant interaction with LVEF subgroups.43 The DELIVER study showed similar results for dapagliflozin.44 These results suggest that both empagliflozin and dapagliflozin would likely also benefit HFpEF patients and should, therefore, be considered.
The panel also voted that MRAs may be considered for HFpEF patients. This was based on the Top Cat trial, which found that spironolactone reduced the risk of HF hospitalisation in HFpEF patients, but was associated with adverse effects such as hyperkalaemia and increased creatinine levels.50 Given the modest benefit from using an MRA in patients with HFpEF with a number-needed-to-treat of 45 and the need to monitor serum potassium and renal function, its use should be carefully discussed with the patient, especially in scenarios where healthcare resources are limited.
A few studies showed that RAS blockade, specifically ARNI, may benefit HFpEF, although the net benefits may be small. The Paragon-HF trial included patients with LVEF ≥45%, HF admission within 9 months or elevated NP levels and estimated glomerular filtration rate ≥30 ml/min/m2. While the study did not find a statistical difference in the primary outcome of HF hospitalisation or CV death, a signal of benefit from sacubitril-valsartan versus valsartan was found in terms of HF hospitalisations in secondary analysis.51 Pre-specified subgroup analyses also showed that certain subsets – those with a lower risk of the primary outcome, such as women and those with an LVEF of 45–57% – significantly benefitted from sacubitril-valsartan.46
The 2022 American Heart Association guidelines noted that in selected patients with HFpEF, the use of ARB may be considered to decrease hospitalisations, particularly among patients with LVEF on the lower end of this spectrum, with a Class 2b recommendation. This was primarily based on the CHARM-Preserved trial, which showed that in HF patients with LVEF >40%, patients treated with candesartan (64.5% with LVEF ≥50%) had a lower risk of HF hospitalisation, which was statistically significant (p=0.047) after covariate adjustment.52 However, two experts highlighted that this analysis was not pre-specified and voted against the use of ARBs in HFpEF patients. Other ARB studies that included HFpEF patients failed to show benefit.53,54 As with MRAs, the modest benefit from an ARNI/ARB in patients with HFpEF necessitates careful patient selection, especially in the context of limited healthcare resources.
Finally, the authors underscore that the underlying cause of HFpEF should be thoroughly investigated and treated to prevent disease progression.
Non-pharmacological Treatment
Statement 21. All patients with HF should be encouraged to enrol in a multidisciplinary care cardiac rehabilitation programme.
Level of evidence: Moderate.
Level of consensus: 100% agree; 0% neutral; 0% disagree.
Statement 22. Patients with LVEF ≤35% after 3 months of achieving OMT should be referred for cardiac implantable electronic devices therapy.
Level of evidence: Moderate.
Level of consensus: 89.5% agree; 10.5% neutral; 0% disagree.
Clinical evidence consistently shows that exercise rehabilitation improves exercise capacity and quality of life in HF patients, including in patients with HFpEF.55,56 Physical conditioning also reduces HF hospitalisations and overall hospitalisations.57,58 Hence, all HF patients should be encouraged to enrol in a multidisciplinary care cardiac rehabilitation programme.
Community-based rehabilitation programmes may help improve the accessibility of and compliance with cardiac rehabilitation in HF patients in the Asia-Pacific region and are associated with favourable CV health benefits, including improved CV markers.59,60
CIED therapy for HF includes the implantable cardioverter defibrillator and cardiac resynchronisation therapy. Randomised trials have demonstrated that the use of CIEDs in carefully selected patients reduces the risk of death across a relatively wide range of clinical scenarios.61–65 However, due to the high cost associated with the use of these devices and the expertise required for patient selection and implantation, a referral to a specialist is recommended. Hence, candidates for CIED – symptomatic HF patients in sinus rhythm with a QRS duration of 130–149 ms and left bundle branch block QRS morphology with EF ≤35% despite 3 months of achieving OMT – should be referred to a specialist as soon as eligible.
Limitations and Conclusion
The 22 recommendations presented in this paper aim to guide clinicians using the most up-to-date evidence. However, given the varied clinical situations and healthcare resources present in the region, these recommendations should not replace clinical judgement. The management of HF patients should be individualised, taking into account the patient’s symptoms, clinical characteristics and comorbidities, as well as patient and caregiver concerns and preferences. Clinicians should also be aware of the challenges that may limit the applicability of these consensus recommendations, such as access to specific interventions and technologies, availability of resources, accepted local standards of care, cultural factors, and one’s own expertise in the management of HF.
Clinical Perspective
- Recent updates in clinical evidence and the differences in the epidemiology of heart failure (HF), as well as the variable availability of healthcare resources between Asian and Western countries warrant the development of consensus recommendations to harmonise the management of chronic HF in the Asian-Pacific region.
- The diagnosis of HF is based on clinical history supported by echocardiographic evidence and natriuretic peptide measurement, if available.
- The authors outlined the recommendations for pharmacotherapy in patients with HF with reduced, mildly reduced and preserved ejection fraction, based on recently released clinical trial evidence.
- All patients with HF should be encouraged to enrol in a multidisciplinary care cardiac rehabilitation programme. Patients with left ventricular ejection fraction ≤35% after 3 months of achieving optimal medical therapy should be referred for cardiac implantable electronic devices therapy.