Renal artery stenosis (RAS) is the most common cause of secondary hypertension, accounting for 1–3% of all cases. This can be secondary to many diseases, including connective tissue disease and vasculitides, with atherosclerosis still being the most common cause in 90% of patients with RAS.1
Haemodynamically significant RAS can result in refractory hypertension, flash pulmonary oedema and end-stage renal disease (ESRD) in long-standing and severe cases. As De Mast and Buetler et al. stated: “Approximately 41% of patients with ESRD have at least one renal artery with more than 50% stenosis.”2 The detection of atherosclerotic RAS may indicate multivascular involvement of the disease. Because it is a window to other vascular beds, a more thorough and comprehensive approach for these patients is important.
Presented here is the case of an elderly female patient with bilateral RAS with atherosclerotic multivascular bed involvement, after bilateral aortoiliac bypass with uncontrolled hypertension, and recurrent heart failure who was successfully treated with bilateral renal artery stenting.
Despite the current controversy regarding the lack of randomised studies to support the benefits of surgical revascularisation over medical therapy for atherosclerotic RAS patients, this case study will serve as new evidence, especially for Asian countries in managing haemodynamically unstable patients with bilateral renal artery stenosis.
Case Report
A 76-year-old woman presented to our institution with a 2-week history of exertional dyspnoea accompanied by bilateral leg swelling. Upon arrival at the emergency department her blood pressure was elevated (180/70 mmHg). Physical examination revealed a distended neck vein, decreased breath sounds and grade 2 bipedal oedema.
Her medical history included insulin-requiring type 2 diabetes, severe peripheral artery occlusive disease and known RAS treated with cilostazol and maintained on multiple medications (amlodipine, clonidine and nebivolol). She was diagnosed with chronic kidney disease (CKD) but was not on dialysis. The patient had a history of coronary artery disease and aortoiliac occlusive disease and had previously undergone coronary artery bypass grafting and aortoiliac bypass grafting. She was a previous 12.5-pack per year smoker.
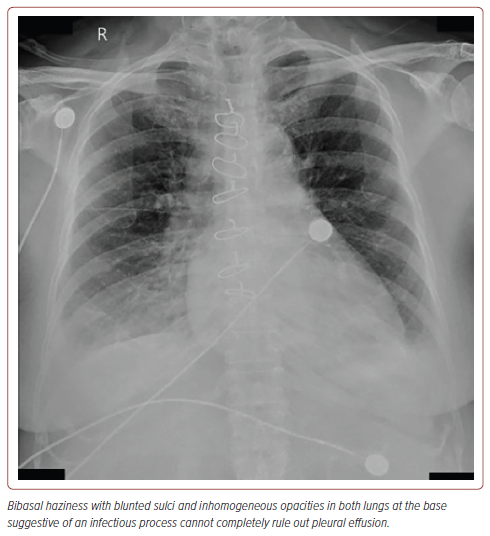
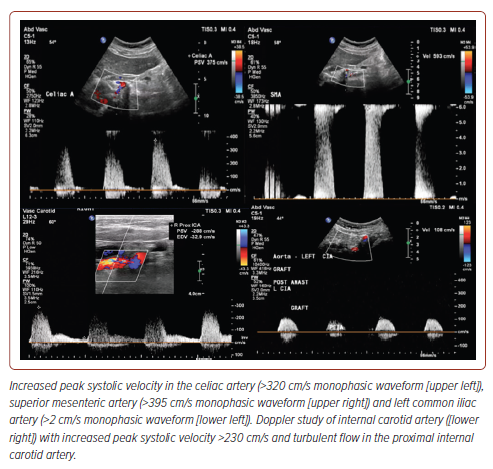
Routine laboratory tests showed a creatinine level of 2.98 mg/dl (estimated creatinine clearance of 15), blood urea nitrogen of 38 mg/dl, and brain natriuretic peptide of 6,097 pg/ml. Chest X-ray revealed bibasal haziness with blunted sulci and inhomogeneous opacities in both lungs suggestive of pneumonia that could not totally rule out effusion (Figure 1). ECG showed normal sinus rhythm, left ventricular hypertrophy by voltage criteria, with late R wave progression. Transthoracic echocardiography showed an ejection fraction of 67.2% using the Simpson method with adequate wall motion and contractility. Abdominal aorta ultrasound was performed for graft surveillance and showed haemodynamically significant atherosclerotic stenoses; more than 70% stenoses in the celiac and superior mesenteric arteries and 50–74% stenosis in the left common iliac artery (Figure 2). The aortobiiliac bypass graft was patent with low flow velocities on the right limb. Renal duplex ultrasound (DUS) showed >60% stenosis in the right renal artery with low flow velocities in the left renal artery. The resistivity indices were both >0.7 in the bilateral renal parenchyma segments. The kidneys showed significant side-to-side differences, with the left kidney being smaller than the right. However, even with an atrophic left kidney, there is evidence of function as depicted by the in vitro estimated glomerular filtration rate, which was noted to be 28 ml/min, with left and right renal function of 50.3% and 49.7%, respectively.
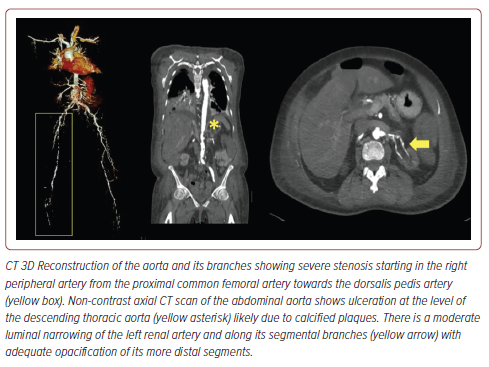
The carotid artery duplex scan (Figure 2) showed 70–99% stenosis in the bilateral internal carotid arteries (CA), >50% stenosis in the bilateral external CA, <50% stenosis in the bilateral common CA, and >50% stenosis in the left vertebral artery (origin to V2). A complete CT aortogram and angiogram of the lower extremities (Figure 3) showed mild narrowing of the right common femoral artery (FA), moderate to severe intermittent narrowing of the right superficial FA, moderate stenosis of the mid to distal deep FA, and mild narrowing of the popliteal, peroneal, anterior and posterior tibial arteries, as well as the dorsalis pedis artery due to calcified and non-calcified plaques.
To further evaluate RAS with the possibility of stenting, CT of the thoracic and abdominal aorta was performed (Figure 3), which revealed heavy intimal calcifications in the aortic cusps, coronary arteries, entirety of the aorta, its proximal branches, and peripheral lower extremities arteries. Ulcerations were observed along the descending thoracic aorta. There was moderate luminal narrowing of the left renal artery, mild luminal narrowing, and its segmental branches likely due to calcified plaques. Calcified plaques were also observed in the proximal right renal artery, causing mild luminal narrowing with adequate opacification of its more distal segments.
Discussion
We report the case of a patient with multiple histories of vascular events presenting with hypertensive crisis accompanied by progressive acute renal failure and flash pulmonary oedema.
Based on the REACH registry, patients with established atherothrombotic disease (EAD) may have polyvascular involvement, and approximately 70% of these have peripheral artery disease.3 EAD patients showed a quasi-linear relationship between the incidence and number of symptomatic arterial involvements. Furthermore, the increase in major vascular events (vascular death, MI, stroke, and other events leading to hospitalisation) was noted to be directly proportional with the number of localisations of atherothrombotic disease (n=601 patients with more than one localisation; 4.1%, 95% CI [0.9–71]; p<0.001). The annual rate of incidence is between 1.8% (isolated) and 4.1% (two or more lesions). Therefore, patients with EAD must be investigated further to detect possible arterial bed involvements, as this may be a good marker for predicting outcomes.
Regarding treatment strategies, routine revascularisation is not indicated in RAS secondary to atherosclerosis because of poor evidence of benefits over medical therapy (class III, level of evidence [LOE] A). However, the European Society of Cardiology (ESC) guidelines recommend revascularisation in various conditions, such as RAS presenting with pulmonary oedema, in which case balloon angioplasty with or without stenting may be considered (class IIb, LOE C).3 The patient underwent percutaneous renal angioplasty using a drug-eluting stent and bare-metal stent (PTRAS) of the bilateral main renal artery.
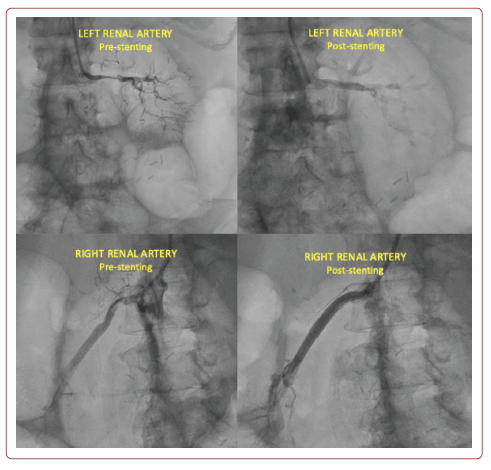
Previous trials, such as ASTRAL and CORAL, and the recent meta-analysis by Riaz et al. have shown that there is no significant difference in the rate of mortality and need for renal replacement therapy between medical therapy and PTRAS.4-6 However, this conclusion was noted to have some limitations, such as the exclusion of patients with small kidney size, moderate stenosis (50–70%), and serious complications that might benefit from revascularisation. Although there were analogous limitations, such as small sample size and low statistical power, several trials have demonstrated that renal vascularisation was irrelevant in patients with Pickering syndrome.7–10 Here we document a patient treated with PTRAS who showed a significantly good clinical outcome. As a result, bilateral angioplasty with stent placement was performed successfully without complications (Figure 4).
Despite surgical intervention, blood pressure control remains the cornerstone treatment for RAS. The ESC guidelines still recommend controlling blood pressure and improving or preserving renal function as the cornerstone treatment for RAS. This includes angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs; class I, LOE B), calcium channel blockers, β-blockers, and diuretics (class I, LOE C) to control blood pressure and to improve and preserve renal function.3 Patients treated with renin–angiotensin–aldosterone system (RAAS) blockers following heart failure guidelines may further decrease renal perfusion pressure and post-glomerular arteriole constriction, which lead to further worsening of renal function; therefore, these patients should be monitored cautiously with mandatory monitoring of serum creatinine.
During the patient’s course in the wards, her intradialytic blood pressure post-intervention showed a significant reduction, averaging 140–150/70–85 mmHg. Nicardipine drip was slowly titrated, and the patient was started with antihypertensive medications. Repeat duplex scan of renal arteries showed normal peak systolic velocities (208 from 245 right; 201 from 88 left) of bilateral origin to distal renal arteries. Repeat blood chemistry test before discharge showed improvement of creatinine to 2.29 mg/dl (endogenous creatinine clearance 29 ml/min/1.73 m2) no longer requiring dialysis.
In terms of prognosis, a multivariate regression analysis was performed by Rocha-Singh et al. from 150 hypertensive patients with 180 renal artery lesions (>75%) who underwent renal artery stenting after 13 months.11 One of the significant variables was a mean arterial pressure >110 mmHg, which predicted blood pressure improvement. A baseline of brain natriuretic peptide >80 pg/ml also demonstrates a significant variable in predicting blood pressure response after successful stent revascularisation. As per the guidelines, life expectancy is reduced in patients with RAS and they may have higher mortality rates if they progress to end-stage CKD. Therefore, close follow-up monitoring is warranted for these patients to maintain adequate blood pressure control and renal function.
Recent strategies have been investigated and used as predictors of future cardiovascular events, although recent studies have shown alternating results. The development of acute complications following revascularisation may be secondary to irreversible microvascular disease of the stenotic lesions due to long-standing hypertension.12 The persistence of elevated pressure may cause glomerulosclerosis and nephrosclerosis, increasing the flow resistance of both kidneys. Because of this pathophysiology, the alteration of this resistance can be used as a marker for structural changes in the renal microvasculature. Radermacher et al. studied 131 patients who underwent renal artery stenting and revealed that patients with a resistive index (RRI) >80 may not benefit from surgical intervention based on blood pressure, renal function, or survival.13 However, a study conducted by Zeller et al. opposed the conclusion that 176 patients with severe RAS had similar reductions in blood pressure and improvement in renal function despite various RRIs.14 In our patient, the RRI ranged from 0.57 to 0.75 (origin to the distal renal artery) before renal artery stenting.
The latest retrospective study by Bommart et al. evaluated the use of renal length and volume alongside RRI in predicting improvement in renal angioplasty.15 Therapeutic response can be predicted with a threshold of renal length-resistive index product of <7 cm (sensitivity of 87% and specificity of 50%), which can be attained via Doppler ultrasound and cortical volume measured with magnetic resonance angiography. Therefore, we suggest determining renal length and weighted volume using the RRI in patients after PTRAS to predict good outcomes.
Overall, a strategy that can be used for the confirmation of Pickering syndrome has been made by Viola et al., featuring renal ultrasonography to determine kidney size with a Doppler assessment of renal velocity and RRIs, and CT of aorta and renal arteries.16 With a cut-off 6–7 cm in longitudinal diameter to predict presence of total occlusion, and RRIs <0.55 among multiple renal artery involvement could support in the diagnosis of a Pickering syndrome to proceed with revascularisation with the use of a minimal contrast media.
Conclusion
RAS is a progressive disease that should be considered a part of the multivascular involvement of vascular disease, rather than a solitary disease affecting only the renal vasculature. The clinical outcomes of patients with atherosclerotic RAS mainly depend not only on the current renal parenchymal condition but also on the atherosclerotic burden with other coexisting cardiovascular diseases.
There are advantages and disadvantages to the use of CT scan versus renal duplex scan in investigating the severity of stenosis of RAS. However, both are complementary, with the renal duplex scan having the advantage of not requiring radiation, not using contrast, and providing good haemodynamic information. It is also best to follow up this patient with a renal artery duplex scan because the stents may interfere with the imaging quality of the CT scan, causing blooming artifacts. However, the downside is that the learning curve of the duplex scan is high, and the results are operator-dependent.
PTRAS is recommended in patients with RAS presenting with heart failure and flash pulmonary oedema to improve outcomes; however, its clinical benefits remain controversial. Most published reports are from developed countries, and this case report may provide good evidence for Asian countries. Recent clinical variables, such as RRI, although not proven, can be used to determine prognostic outcomes in terms of blood pressure control and improving renal function.
Previous studies have shown no significant difference in the mortality rate between medical therapy and PTRAS. Here, we documented a patient treated with PTRAS who showed excellent clinical outcomes.
Clinical Perspective
- The need to have a close suspicion in diagnosing Pickering syndrome is warranted in patients with a history of renal artery stenosis (RAS) on a background of multiple vascular involvement presenting with uncontrolled hypertension, worsening of kidney function, and flashed pulmonary oedema.
- Renal duplex ultrasound with Doppler may be used for diagnosis given a specific cut-off resistive index as well as CT scan of the aorta and renal artery which may contribute to the confirmation of Pickering syndrome and to support for the need of revascularisation.
- Percutaneous transluminal renal angioplasty may be performed as the treatment for RAS in patients with Pickering syndrome on top of medical management.