Introduction
Tetralogy of Fallot (TOF) is one of the most common types of adult congenital heart disease.1 Progress and innovation in the diagnosis and management of TOF have led to dramatic improvements in early survival. Unfortunately, the surgical management of TOF is not considered curative; many patients have late complications, such as arrhythmias and cardiac failure, and frequently need appropriate follow-up and reintervention.2–4 Most patients develop pulmonary regurgitation (PR) or right ventricular outflow tract stenosis (RVOTS), which can induce right ventricular (RV) dilatation, ventricular tachycardia (VT) and sudden death.5 Reoperation, especially RV outflow reconstruction with or without cryosurgery, can reduce RV volume and the incidence of VT and, possibly, sudden death.6–9 Many reports have described indications for reoperation of TOF, which are based on QRS duration, RV volume and functions, and exercise capacity; however, there is no consensus on the indications and optimal timing of reoperation for patients with TOF.6,8,10–12 In addition, recent advances in percutaneous pulmonary valve replacement/implantation have offered less invasive options for TOF patients, potentially allowing for earlier treatment.13,14 Yet, challenges persist in percutaneous treatments, such as the need for concurrent procedures for tricuspid valve intervention, and the prevalence of pulmonary valve stenosis and RVOTS, especially in Japan and other Asian countries. This underscores the importance of evaluating data specific to the Japanese population.15 Furthermore, considering the potential for future reoperations, examining data from the pre-percutaneous treatment era becomes crucial.
We undertook this study to gather and analyse data from adult TOF patients who underwent reoperation in Japan, aiming to understand the practical and clinical indications and outcomes of these procedures.
Methods
Study Design and Patient Population
We retrospectively collected data from adult patients with TOF who underwent reoperation at experienced surgical centres from 2000 to 2015. The exclusion criteria included: pulmonary atresia with ventricular septal defect (VSD) repaired by an extracardiac conduit, double-outlet right ventricle with subaortic VSD and pulmonary stenosis and pulmonary atresia. We determined experienced surgical centres where >10 reoperations were performed by using a previously conducted national survey.15 Ethical approval was obtained from the research ethics committee of each institution.
Data Collection
Each patient’s data included the patient’s profile (age, past medical history, which included initial intracardiac repair, and physical data such as height and weight), reoperation data (including indication, date and operation methods), perioperative examinations (which encompassed ECG measurements including QRS duration, chest X-ray with preoperative cardiothoracic ratio, echocardiography, MRI, catheterisation, laboratory data and cardiopulmonary exercise test [CPX]) and the patient’s outcome. The main indications for reoperations were categorised by clinical physicians at each institution as PR, RVOTS, VSD, atrial septal defect (ASD), aortic regurgitation (AR), aortic dilatation and tricuspid regurgitation (TR).
In our analysis, we compared a comprehensive array of variables obtained from a comprehensive set of assessments, including MRI, catheterisation data, CPX results, blood laboratory tests (such as B-type natriuretic peptide levels) and CT measurements of aortic dimensions.
We collected the outcomes such as death, infective endocarditis, re-reoperation and admissions for heart failure and stroke after reoperation.
Statistical Methods
Categorical variables are presented as numbers and proportions, while continuous variables are presented as mean ± SD or median with the interquartile range (median [Q1–Q3]). For preoperative evaluation, the number of cases per year was categorised into tertiles (Q1–Q3) to avoid linearity assumptions. All statistical analyses were performed using R (version 4.2.2, R Foundation).
Results
Study Patients
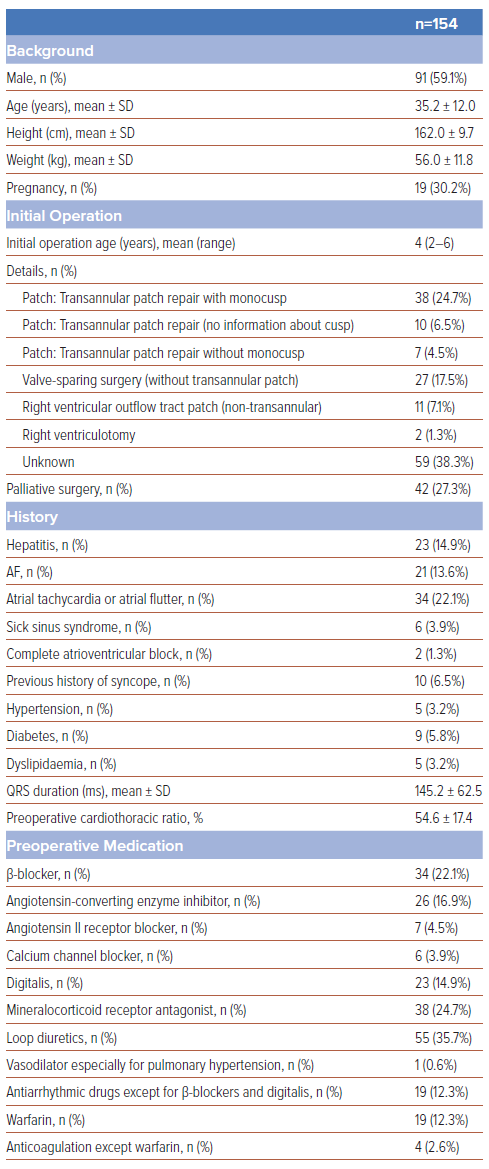
A total of 154 patients (59.1% male, aged 35.2 ± 12.0 years) from 10 hospitals were enrolled. Baseline characteristics are detailed in Table 1. The initial operation was performed at a median age of 4 years. Unfortunately, we could obtain details of initial operation for RV repair in only 61.7% of enrolled patients, and the indication for the initial surgery was unknown in the remaining patients. About one-third of women (30.2%) had experienced pregnancy before their reoperations. Of the patients, 14.9% had hepatitis and arrhythmia; 13.6% had AF, 22.1% had atrial tachycardia or atrial flutter, 3.9% had sick sinus syndrome and 1.3% had complete atrioventricular block, while 6.5% had experienced syncope. As these patients were young, lifestyle-related diseases, such as hypertension, diabetes and dyslipidaemia, were not frequent (3.2%, 5.8% and 3.2%, respectively). Preoperative medications varied, with diuretics being the most frequently used type preoperatively (35.7%).
Indications for Reoperation
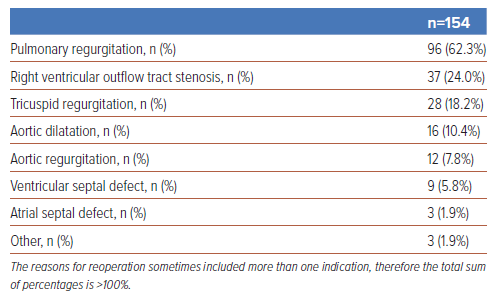
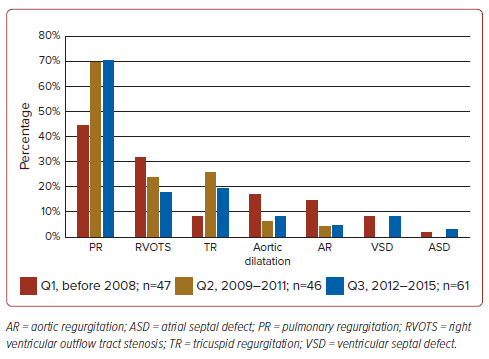
Table 2 presents the chief indication for reoperations among the enrolled patients. The chief indication for reoperation was PR (62.3%), followed by RVOTS, TR, aortic dilatation, AR, VSD, ASD and other (24.0%, 18.2%, 10.4%, 7.8%, 5.8%, 1.9% and 1.9%, respectively). Figure 1 illustrates the time trend of reoperation indication, which shows an increasing percentage of PR and a decreasing percentage of RVOTS.
Preoperative Evaluation
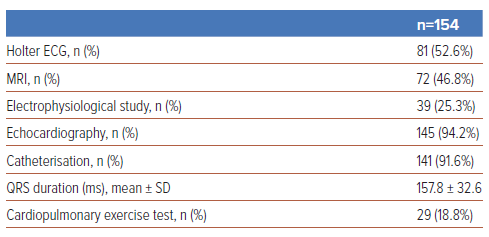
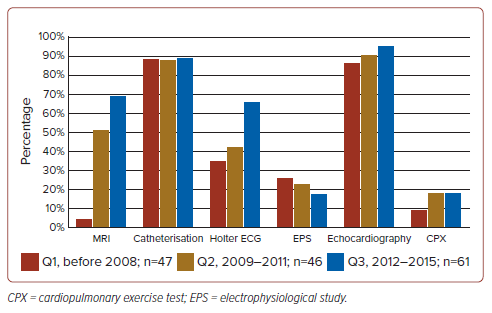
We performed echocardiography and catheterisation in the majority of patients (94.2% and 91.6%, respectively) before reoperation; however, an electrophysiological study (EPS) and CPX were performed only in 23.5% and 18.8% of patients, respectively (Table 3). Figure 2 shows the time trend of preoperative evaluations, which indicates markedly increasing frequencies of MRI and Holter ECG in 2012–15, compared with the period before 2008 (increasing from 6% to 72% and from 38% to 69%, respectively).
Nearly all patients were classified as New York Heart Association (NYHA) class I–II (29% in NYHA class I; 63% in NYHA class II; 6% in NYHA class III; 2% in NYHA class IV). Preoperative values are described in Supplementary Table 1. In patients with a reoperation indication of PR, RV end-diastolic volume index was 183.0 ± 59.3 ml/m2, RV end-systolic volume index was 101.3 ± 47.8 ml/m2 and RV ejection fraction was 45.1 ± 11.2%. In patients with a reoperation indication of RVOTS, the RV systolic pressure was 85.1 ± 23.4 mmHg, trans-RVOT gradient was 46.3 ± 30.8 mmHg and RV systolic pressure:systolic aortic pressure ratio was 0.7 ± 0.2. In VSD or ASD patients, the pulmonary blood flow:systemic blood flow (Qp:Qs) ratio was 1.8 ± 0.6. In patients with aortic dilatation CT measurements included 26.5 ± 4.8 mm of aortic valve diameter, 45.8 ± 14.7 mm at the sinotubular junction and 38.8 ± 11.5 mm for the ascending aorta.
Outcome
During the mean follow-up period of 5.7 years (range 2.5–8.0 years), there were eight deaths (5.2%). Three patients (1.9%) had strokes, six (3.9%) were hospitalised for heart failure and four (2.6%) developed infective endocarditis. Re-reoperation was needed in 11 (7.8%) patients. New-onset VT and AF/atrial flutter developed in two (1.3%) and two (1.3%) patients, respectively. Significant bradycardia, including sick sinus syndrome and complete atrioventricular block, occurred in seven (4.5%) patients. Long-term mortality did not significantly vary among each chief reason of reoperation (PR, RVOTS and TR).
Discussion
This is the first and largest nationwide study on the clinical indications for reoperation in adult patients with repaired TOF. According to some reports published about the indications for reoperation, the reoperation indications in this study slightly differ from those in other countries.4,16 A previous nationwide questionnaire survey on repaired TOF had shown that RVOTS was the most frequent reason for reoperation in Japan.15 This study shows that the chief indications for reoperation in patients with TOF have shifted from RVOTS to PR, and the current outcome after reoperations for TOF patients was comparable with that in previous reports from other countries. While reoperation indications are similar to those in previous studies, they vary among patients. Furthermore, the outcome of reoperation is generally positive, but re-reoperation was required in 7.1% of patients.
Pulmonary Regurgitation: The Chief Indication for Reoperation
RVOTS (31.7%) had been considered the most common indication for reoperation in Japan compared with PR (29.0%).15 This study has shown that the major reoperation indication was PR (62.3%) compared with RVOTS (24.0%). More than three-quarters of reoperations were related to residua and sequelae of the RVOT, which is similar to previous reports.17 To discuss this shift we should consider the intracardiac operation technique.
In western countries, transannular patch repair was prominent in the early 1970s.18,19 However, this repair is associated with significant PR, which may induce RV dilatation/dysfunction, VT and sudden death. Transatrial–transpulmonary approaches were revisited in the 1990s. In contrast, in Japan, the standard procedure of repair during the early 1970s was to excise the obstructive RVOT muscle through a ventriculotomy and patch closure of the VSD.20,21 In a Japanese multicentre study, transannular patch use was applied in only 13% of patients with total repaired TOF in 1972 and 63% in 1982.4 This approach, which Kurosawa named ‘conotruncal repair’, is different from previous operations in other countries: small RVOT incision (similar to the ‘no’ or ‘short’ ventriculotomy) with small ventricular patch.22 These methods were thought to result in a favourable outcome; only 4–5% of late cardiac deaths and 0–6% reoperation rates 20 years after intracardiac repair were observed.4 Therefore, in Japan, this transannular patch repair is even now considered as the gold standard for intracardiac repair for patients with TOF. Considering these histories, we could speculate two reasons why the indication of reoperation had shifted from RVOTS to PR in Japan.
First, our strategies with transannular patch repair with small incision might be associated with slow progression of PR. The lesion was relatively smaller; therefore, the onset of regurgitation might be later than in other countries. Long-lasting PR, even if it is mild to moderate, could induce RV dilatation/dysfunction in patients with repaired TOF. So, gradually PR has become the primary indication of reoperation even in Japan.
Second, we found RVOTS to be the primary indication of reoperation, which was confirmed by our previous questionnaire.15 This experience might help us to avoid residual RVOTS formation and a more rigorous resection of RVOTS.4 However, we could not confirm this hypothesis from the results of this study. Considering our results, MRI and other modalities might be mandatory for evaluation of reoperation for PR in patients with repaired TOF.
Other Indications for Reoperation
About 20% of patients had TR as an indication for reoperation, challenging the necessity of tricuspid surgery in RVOT issues.23,24 Residual VSD was lower at 5.8%, suggesting improved surgical techniques.25 Aortic issues accounted for 10.4% (regurgitation) and 7.8% (dilatation) of reoperations, with antihypertensive medication use possibly influencing lower reoperation rates for aortopathy.26,27 Notably, aortic root dilatation (>40 mm) was significant in 15% of TOF cases, underscoring the need to monitor and possibly intervene in left heart lesions.28
Preoperative Assessment
QRS duration has traditionally been a straightforward indicator for reoperation. However, the advent of advanced imaging and physiological testing now affords a more nuanced understanding of cardiac structure and function.5 Notably, cardiac MRI, heralded by Geva as the definitive method for evaluating RV performance and PR severity, saw its preoperative use increase from 6% before 2008 to 72% between 2012 and 2015.6 This uptick corresponds with the growing recognition of PR as a primary reason for reoperation. While CPX provides critical insights for reintervention strategies and EPS is linked to ventricular volume considerations, their application was limited to 23.5% and 18.8% of cases, respectively, in our study.29 The high documentation rates for echocardiography and catheterisation (94.2% and 91.6%, respectively) contrast with the less frequent use of CPX and EPS, which is thought to be due to their complexity and invasiveness. Despite expectations that echocardiography would be performed in all cases, it was officially recorded in only 94.2% of them, indicating a potential for incomplete documentation, particularly in point-of-care diagnostics. This is not anticipated with catheterisation or in the utilisation of CPX and EPS. The lower implementation rate of CPX and EPS does not necessarily imply their ineffectiveness in preoperative reoperation decision-making compared to other modalities, but rather suggests that effort and resource allocation are also considered. Further research is warranted to explore this aspect.30
Details of Reoperation Indications
Our study shows that outcomes after reoperation for repaired TOF align with previous findings, showcasing 92% and 95% 5-year survival rates reported by Therrien et al. and Discigil et al., respectively.31,32 Despite including a broader range of indications beyond pulmonary valve replacement, outcomes for RVOTS and TR were similar, validating our indication criteria. The incidence of new-onset VT and AF/atrial flutter was about 1.3%, lower than that reported internationally, but consistent with Nakazawa et al.’s findings in Japan, indicating a higher VT risk but no significant increase in atrial arrhythmia risk post-reoperation.33,34 The occurrence of sick sinus syndrome or atrioventricular block post reoperation (4.5%) underlines the necessity for careful postoperative monitoring. Re-reoperation rates were 7.1%, slightly above the 5-year redo rate for pulmonary valve replacement of 4.9%, reflecting our inclusive study cohort.35
Variability in reoperation indications, especially for PR, suggests no strict consensus on cut-off values for cardiac functional parameters. Our data show considerable preoperative evaluation variation, with an RV end-diastolic volume index average of 183.0 ± 59.3 ml/m2 among patients, suggesting larger volumes compared to prior studies. This variability extends across different reoperation indications, yet our outcomes lend indirect support to our chosen criteria.6
Study Limitations
To our knowledge, this is the first nationwide survey of reoperation in patients with TOF. However, there are several limitations. First, the retrospective nature of the study meant that not all patient data were available. In particular, operative details from previous surgeries were not available for approximately one-third of the patients, which significantly impacts the completeness of our data set. Despite this, we were able to achieve our aim of revealing the trend of reoperation indications and the details of the preoperative examination. Second, the study only included 154 patients, limiting the ability to perform a detailed comparison of patient characteristics and reoperation indications. While we can provide suggestions for reoperation clinical decision-making, these are based on a relatively small sample and should be viewed as preliminary. Lastly, evaluations of each indication for reoperation were based on the examination results from each institution, which could lead to variability in the criteria for reoperation among institutions. This heterogeneity is an inherent limitation of retrospective studies and should be taken into account when interpreting the conclusions drawn from our data.
Conclusion
The number of reoperations in adult patients with TOF has been increasing yearly, and the indication had shifted from RVOTS to PR, especially in Japan. Surgical decisions, especially with concurrent indications, need adaptable approaches. Our findings, despite the absence of a consensus on preoperative evaluation and thresholds for reoperation, may support current surgical decision-making.
Clinical Perspective
- The primary indication for reoperation for adults with tetralogy of Fallot has shifted from right ventricular outflow tract stenosis to pulmonary regurgitation.
- There is increased preoperative use of cardiac MRI for evaluating pulmonary regurgitation severity.
- There is significant need for reoperation due to tricuspid regurgitation, requiring careful management.
- Variability in reoperation indications highlights the need for consensus on evaluation thresholds.