Left ventricular thrombus (LVT) is a well-known complication of acute MI (AMI) and non-ischaemic cardiomyopathies.1 The presence of LVT increases the risk of embolic complications, such as stroke or systemic embolisation, hence treatment with oral anticoagulation is often indicated.2 The European and American guidelines recommend a period of anticoagulation in patients with AMI and LVT.3,4 Currently, the oral anticoagulant (OAC) of choice is warfarin, a vitamin K antagonist (VKA). More recently, however, there has been increasing evidence to support the use of direct oral anticoagulants (DOACs), with several studies showing comparable efficacy and safety between them.5–13
Although the initial treatment options for LVT are better established, the management of patients after the initial duration of anticoagulation is more complex and varied. After a period of initial anticoagulation, subsequent repeat imaging is often done via transthoracic echocardiogram (TTE) with/without contrast and occasionally cardiac MRI (CMR) to assess for resolution of LVT.2 The rate of resolution of LVT following anticoagulation therapy varies widely, ranging from 48.1% to 91.7%, with a recurrence rate after anticoagulation of as high as 18.5%.5,7,14 Currently, in the AMI setting it is common practice to continue anticoagulation if the thrombus persists, and to potentially stop OAC and resume antiplatelet therapy if the thrombus resolves.15 However, patients with LVT often have a depressed LV ejection fraction (LVEF) and/or large akinetic/dyskinetic areas, particularly apical, which predispose to recurrent LVT formation, especially if these risk factors persist.16 Thus, the decision to continue or stop OACs in the absence of LVT following initial anticoagulation is not as straightforward.
This comprehensive literature review aims to synthesise the currently available evidence, provide recommendations for the initial pharmacological therapy for LVT and duration of treatment, and provide guidance on the subsequent treatment options after the initial period of anticoagulation.
Methods
A comprehensive search was performed of studies from inception until 1 July 2022 on the following electronic databases: MEDLINE (Ovid), Embase (Ovid), Scopus, and Google Scholar. The search consisted of the keywords ‘left ventricular thrombus’, ‘left ventricular clot’, ‘treatment’, ‘management’, and synonyms. The full search strategy can be found in Supplementary Material Appendix I. The retrieved papers were then exported into the systematic review managing software Covidence (Veritas Health Innovation), where duplicates were removed. The inclusion criterion was a report on the management of LVT. The exclusion criteria were a focus on paediatric populations, individual case reports, other reviews, and studies published in languages other than English. The titles and abstracts were initially screened, followed by an evaluation of the full text of the articles for relevance. The references of included studies were also subsequently screened to identify other potential studies. Data extracted from the papers consisted of publication details, patient characteristics, management options and outcomes.
Results
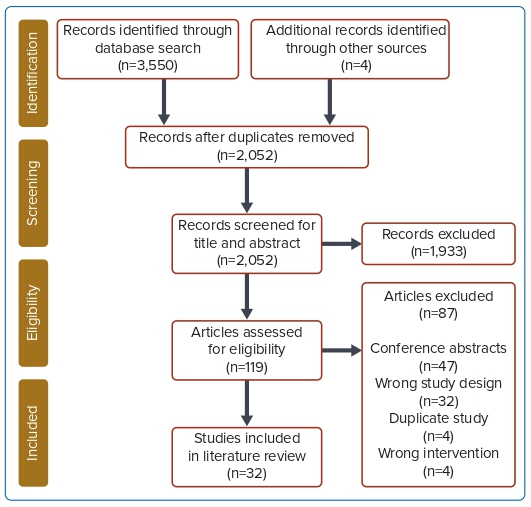
A total of 32 studies were included in the final analysis.13,16–46 Figure 1 shows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart. Twenty-three studies (71.9%) were retrospective cohort studies, three studies (9.4%) were case series, three studies (9.4%) were randomised controlled trials (RCTs), two studies (6.4%) were prospective cohort studies and one study (3.1%) was a non-randomised, open-label trial. An overall summary of all studies included is given in Supplementary Material Appendix II.
Aetiology
The most common underlying causes of LVT were AMI in 14 studies (43.8%), ischaemic cardiomyopathy in 13 studies (40.6%) and takotsubo cardiomyopathy in one study (3.1%). Four studies (12.5%) did not record aetiology.
Initial Anticoagulation Strategy
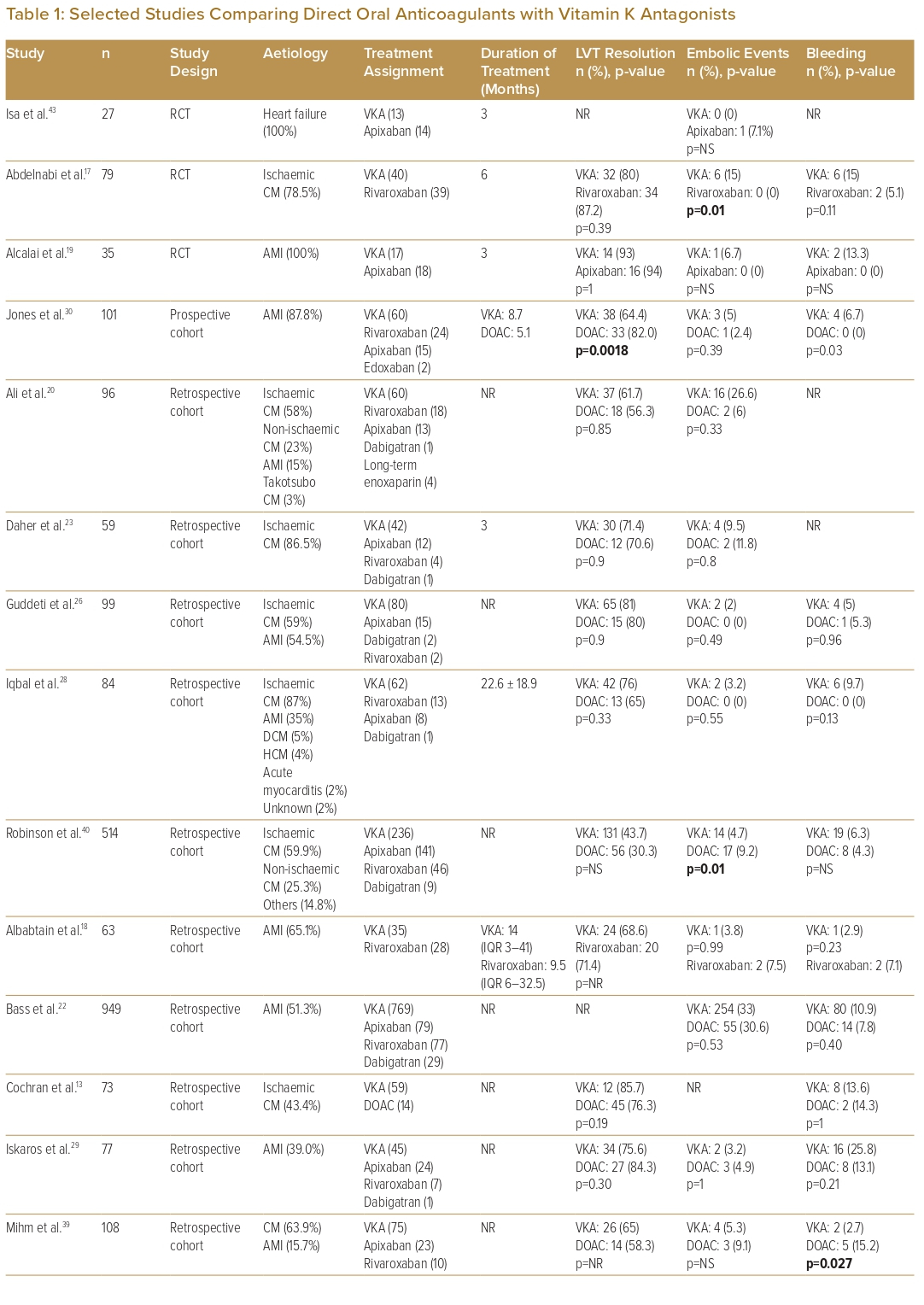
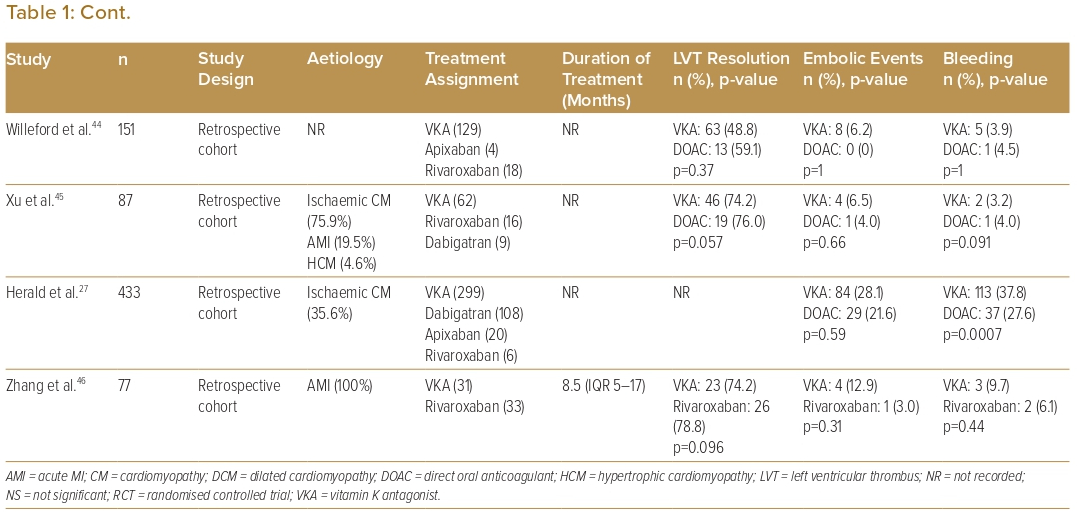
The initial anticoagulation strategy included both DOACs and VKAs in 21 studies, VKA only in seven studies, DOACs only in three studies, and was not recorded in one study. Of these studies, two studies each also had patients on heparin and anti-platelets only as initial coagulation. Eighteen studies compared VKAs with DOACs (Table 1). For studies that used DOACs, 66.7% included more than one type of DOAC (from among apixaban, dabigatran, rivaroxaban or edoxaban), 15.6% used rivaroxaban only and 6.3% used apixaban only. Of the limited number of studies available for analysis, the majority showed similar outcomes between VKA and DOAC in terms of rates of LVT resolution, embolic events and bleeding events. One study showed superior LVT resolution with DOACs, and one study each showed fewer embolic events with warfarin and DOACs, respectively, two studies showed fewer bleeding events with DOACs, and one study showed fewer bleeding events with warfarin.17,27,30,39,40
Eight studies each (25.0%) described a median duration of OAC treatment of between 3 and 6 months, seven studies (21.9%) described a treatment duration of 6–12 months and seven studies (18.8%) described a median treatment duration of >12 months.16–19,21,23,24,28,30,32–
37,41,43,46 The median time to LVT resolution ranged from 2 to 3.4 months for DOACs and from 4 to 9 months for VKAs, with 70–86% of patients on DOACs and 49–76% of patients on VKAs achieving LVT resolution by 12 months.13,30,46 Five studies found a significantly shorter time to resolution with DOACs compared with VKAs. One study noted significantly fewer bleeding complications with the use of dual antithrombotic therapy (DAT) consisting of VKA with aspirin or clopidogrel, as compared with triple antithrombotic therapy (TAT), which consisted of VKA with aspirin and clopidogrel.24 In patients who require TAT due to persistent risk of thromboembolism, close follow-up is needed to identify any bleeding complications.46
Management after Initial Anticoagulation
Five studies repeated imaging less than 3 months after initial imaging, 10 studies repeated imaging between 3 and 6 months after initial imaging, and four studies repeated imaging more than 6 months after initial imaging.16–19,21,23,24,26,28–33,35,37,39–41 All studies used TTE with or without contrast as the imaging modality of choice, with select patients undergoing CMR.
Thrombus Persistence
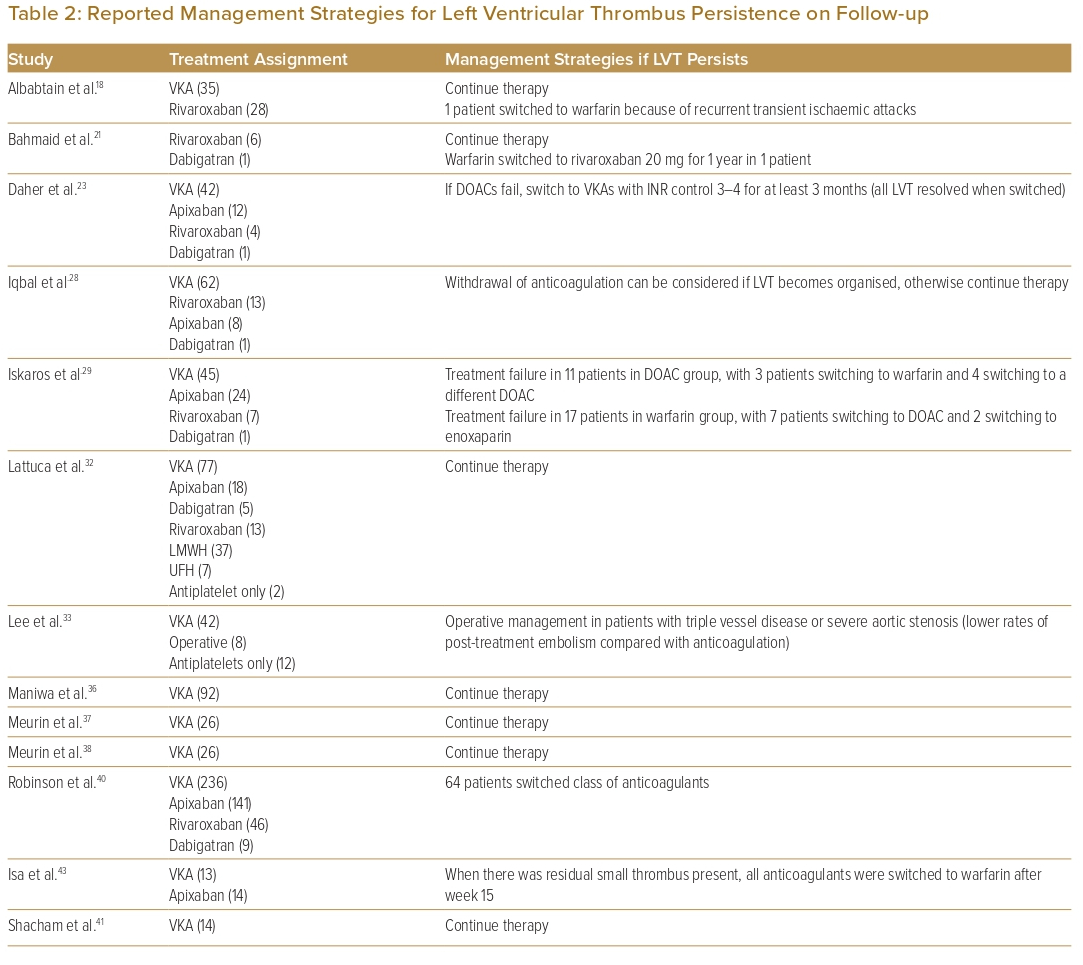
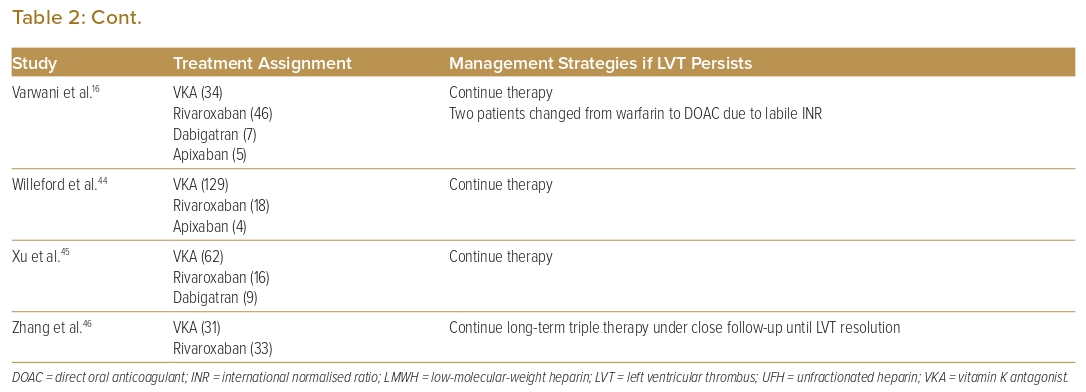
Seventeen studies that reported on management strategies after follow-up echocardiography showed non-resolution of LVT (Table 2). Eleven of these studies (64.7%) reported that therapy continued.16,18,21,32,36–38,41,44–46 Six studies (37.5%) reported switching one anticoagulant class for another (i.e. DOAC for warfarin or vice versa) due to treatment failure.16,18,21,23,29,40 One study had a protocol whereby treatment failure with DOACs was switched to warfarin for 3 months with a higher target INR (international normalised ratio) of 3–4 as opposed to a target INR of 2–3 as recommended, and which achieved a 100% resolution rate in all patients who switched.23 One study used operative management (i.e. thrombectomy, Dor procedure) in patients with concomitant indications for surgery such as triple-vessel disease or severe aortic stenosis.33
Thrombus Resolution
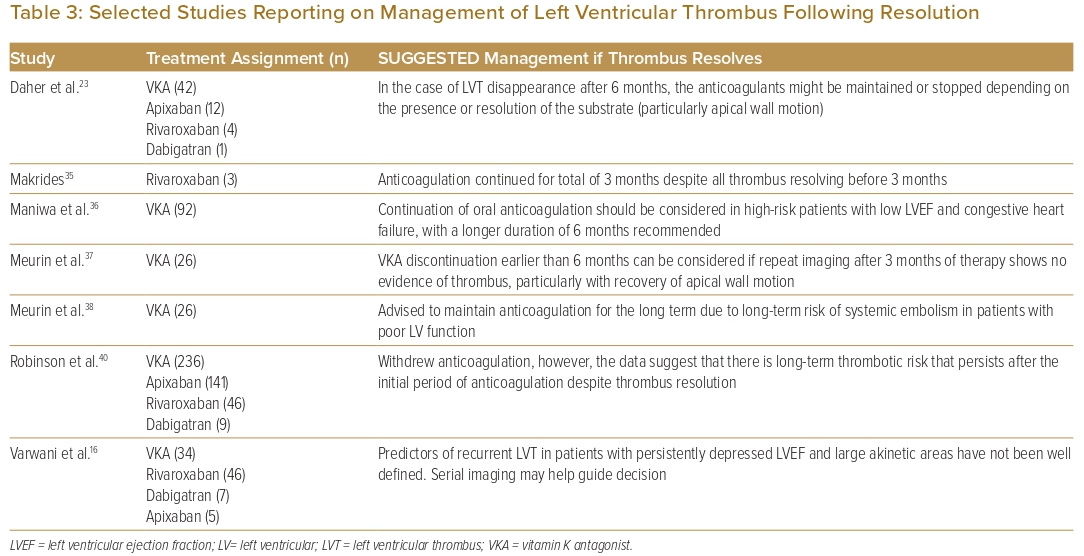
Most studies (60%) reported withdrawal of OAC after thrombus resolution on repeat imaging. Seven studies proposed management strategies following LVT resolution (Table 3). Three studies suggested that continuing OAC should be considered in patients with impaired wall motion or large akinetic areas, or in patients with low LVEF or severe congestive heart failure.16,23,36–38 One study suggested stopping OAC earlier than at the proposed 6 months if repeat imaging at 3 months showed LVT resolution with recovery of apical wall motion.37
Discussion
Aetiology and Pathophysiology
In the majority (84%) of the studies included in this review, the most common aetiology of LVT was ischaemic heart disease. Other aetiologies recorded include dilated cardiomyopathy (DCM), hypertrophic obstructive cardiomyopathy and takotsubo cardiomyopathy.20,28,31–33,45 This could be significant, given that the different pathophysiologies of the differing aetiologies may influence the formation of LVT, which could have implications for therapy. For instance, endocardial injury and inflammation may be dominant factors in AMI, but stasis may be the dominant factor in DCM.47 Despite differing aetiologies, LVT due to ischaemic and non-ischaemic cardiomyopathies is treated similarly with anticoagulation.16,20,28,31,32,40 The 2022 American Heart Association (AHA) statement similarly recommends the use of OAC for 3 months in LVT due to AMI, and OAC for 3–6 months in patients with LVT due to DCM, and repeat imaging after initial duration of treatment.47
Initial Anticoagulation Strategy
The 2017 European Society of Cardiology guidelines recommended up to 6 months of anticoagulation if LVT is detected following AMI.3 The 2013 American College of Cardiology Foundation/AHA ST-elevation MI (STEMI) guidelines recommend as reasonable (class 2a, level c evidence) 3 months of VKA therapy for patients with STEMI and asymptomatic LVT with a target INR of 2–2.5 when combined with dual antiplatelet therapy (DAPT).4 The 2022 AHA scientific statement recommends treatment of post-MI LVT with an OAC for 3 months, for which DOACs are a reasonable alternative to warfarin.47
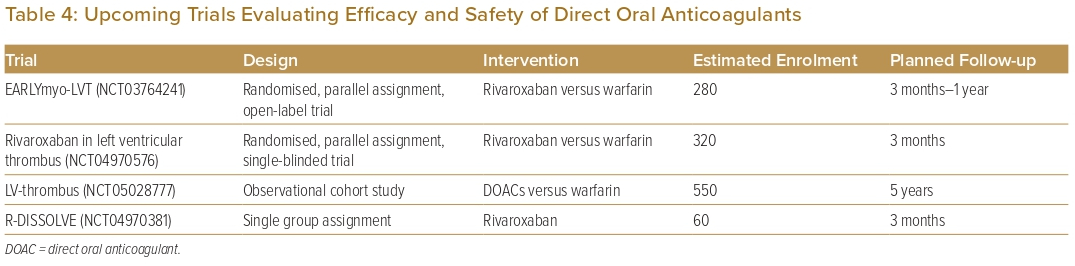
Comparison of the outcomes of initial anticoagulation options for LVT has been reported, with numerous observational studies and a limited number of small RCTs comparing DOACs with VKAs (Table 1). A large retrospective observational study by Robinson et al. involving 514 patients found that DOACs were associated with a significantly higher risk of stroke or systemic embolism.40 However, observational studies lend themselves to inherent bias and, therefore, cannot be used to formulate guidance on treatment strategies. Furthermore, to date, there are only three reported RCTs on the topic: the No-LVT trial, which randomised 79 patients to either rivaroxaban or warfarin; the study by Alcalai et al., which randomised 35 patients to apixaban or warfarin; and the study by Isa et al., which randomised 27 patients to apixaban or warfarin.17,19,43 These studies did not find a significant difference in terms of stroke, systemic embolism or major bleeding.17,19,43 Several meta-analyses have similarly found no significant difference in clinical outcomes.5–13 In addition, several observational studies have suggested that DOACs may be associated with a faster rate of resolution compared with warfarin.18,20,29,30,46 Thus, DOACs may be a reasonable alternative to warfarin, but larger RCTs are needed to validate this statement. There are two currently recruiting RCTs and two other observational studies that aim to further evaluate the efficacy and safety of DOACs versus warfarin in the treatment of LVT (Table 4). Before the results of these RCTs become available, we recommend warfarin as the standard of care due to its larger evidence base, with DOACs as a reasonable alternative, especially if there are issues with monitoring or maintaining therapeutic INR.
Concomitant Antiplatelet Therapy
Pertinent to this discussion is the issue of adjunctive antiplatelet therapy, on top of anticoagulation for LVT resolution. Most patients in the studies included were on concurrent antiplatelet therapy, with a significant number on DAPT, given that the majority of patients with LVT often have concurrent indications for antiplatelet therapy (e.g. MI, ischaemic heart disease). The decision to use a single or dual antiplatelet strategy depends on the patient’s bleeding risk, the strength of the indication for antiplatelet therapy, and the anticoagulant used.3 A retrospective study of 83 patients comparing the safety of DAT (consisting of warfarin with aspirin or clopidogrel), with that of TAT (warfarin with aspirin and clopidogrel), found a significantly increased risk of all-cause mortality, thromboembolic events, rehospitalisations for MI or heart failure and any bleeding at 1 year in the TAT group, with bleeding complications being the most significant.24 However, that study did not evaluate the efficacy of LVT resolution rates. Other trials that evaluated DAT versus TAT in patients with AF after percutaneous coronary intervention found DAT to be just as efficacious as TAT in terms of prevention of thromboembolic and major adverse cardiovascular events, with a significantly lower bleeding risk.48,49 Consequently, triple therapy for longer durations should be used only after careful consideration with close monitoring for bleeding complications. In patients with a strong indication for DAPT, several trials and observational studies have suggested that DOACs are a safer choice than warfarin.16,48–51 In patients requiring DAT or TAT, use of less potent antiplatelet agents such as aspirin or clopidogrel (versus ticagrelor) may be considered to reduce the bleeding risk.
Choice of Imaging Modality for LVT
Most studies repeated initial imaging within 3–6 months after initiation of anticoagulation, which corresponded closely to the median time to LVT resolution reported.18,29,32 All studies chose TTE (with/without contrast) as the imaging modality of choice. CMR may be considered if TTE is negative and there is a high clinical suspicion of persistent LVT, however, it is unclear whether treating LVT detectable only on CMR leads to improved outcomes.52 The 2022 AHA scientific statement recommends repeat imaging at 3 months with the same modality of imaging (or more detailed) that was used to initially diagnose the LVT.47 In the case of LVT persistence, thrombus morphology could be better assessed with contrast TTE, which improves the endocardial border definition, or CMR, which is considered the gold standard imaging technique in assessing the presence, size and location of LVT.53
Management Options for LVT Persistence
In large observational studies, the rate of thrombus resolution at 6 months ranged from more than 80% to as low as 30%.26,40 In the setting of persistent LVT, most studies continued anticoagulation or switched to a different anticoagulant (Table 2). In a retrospective study by Daher et al., LVT resolution rates were similar with both VKAs (INR target 2–3) and DOACs (71.5% versus 70.6%, p=0.9). Patients without LVT resolution on DOACs were switched to warfarin with a higher target INR of 3–4 for at least 3 months (n=5/17), and patients who switched all achieved LVT resolution.23 Other studies also recommended switching anticoagulation therapies when there was non-resolution of LVT, either from DOACs to VKAs or vice versa, or even switching to a different type of DOAC.16,29,43 On the basis of consensus opinion, the 2022 AHA statement recommended switching to an alternative OAC or low-molecular-weight heparin when initial therapy fails.47
Another factor to take note of is the morphology of LVT on TTE. Iqbal et al. reported that organised thrombus without high-risk features (non-mobile, non-protruding) was associated with a low rate of embolisation, allowing for possible safe withdrawal of anticoagulation despite persistent thrombus.28 The 2022 AHA statement stated that discontinuation of OAC in patients with persistent mural thrombus is not unreasonable, especially if the thrombus is calcified or organised.47
In the absence of other concomitant indications for surgery (e.g. coronary artery bypass, valve surgery etc.), there is insufficient available data to recommend surgical options solely for LVT management and this should be evaluated carefully on a case-by-case basis.47
Management Options after Thrombus Resolution
Most studies reported withdrawal of anticoagulation after LVT resolution. However, even with resolution of LVT, there is a persistent risk of thromboembolism.15 Some of these features can be evaluated on TTE and used to guide subsequent management. Observational studies found that impaired LV wall motion or large akinetic areas, or a significantly depressed LVEF were associated with a greater thromboembolic risk, even after LVT resolution.16,23,36–38 Blood stasis identified on CMR may also predict occurrence of embolic events after AMI and anticipate the recurrence of LVT after anticoagulation cessation.54,55 Prolonged antithrombotic therapy appears reasonable, especially if the LVEF or wall motion remains severely impaired.23,32,36 Alternatively, Daher et al. proposed that anticoagulation withdrawal earlier than 6 months can be considered if repeat imaging after 3 months shows resolution of thrombus with recovery of apical wall motion.23
Upon cessation of anticoagulation, the decision for antiplatelet therapy should be based on traditional clinical indications. Current reviews recommend the completion of 12 months of DAPT after cessation of anticoagulation therapy following AMI.15 In non-AMI patients, single antiplatelet therapy may be given in the presence of recommended conditions (i.e. coronary artery disease, stroke, peripheral vascular disease).28
A Practical Approach to Managing LVT
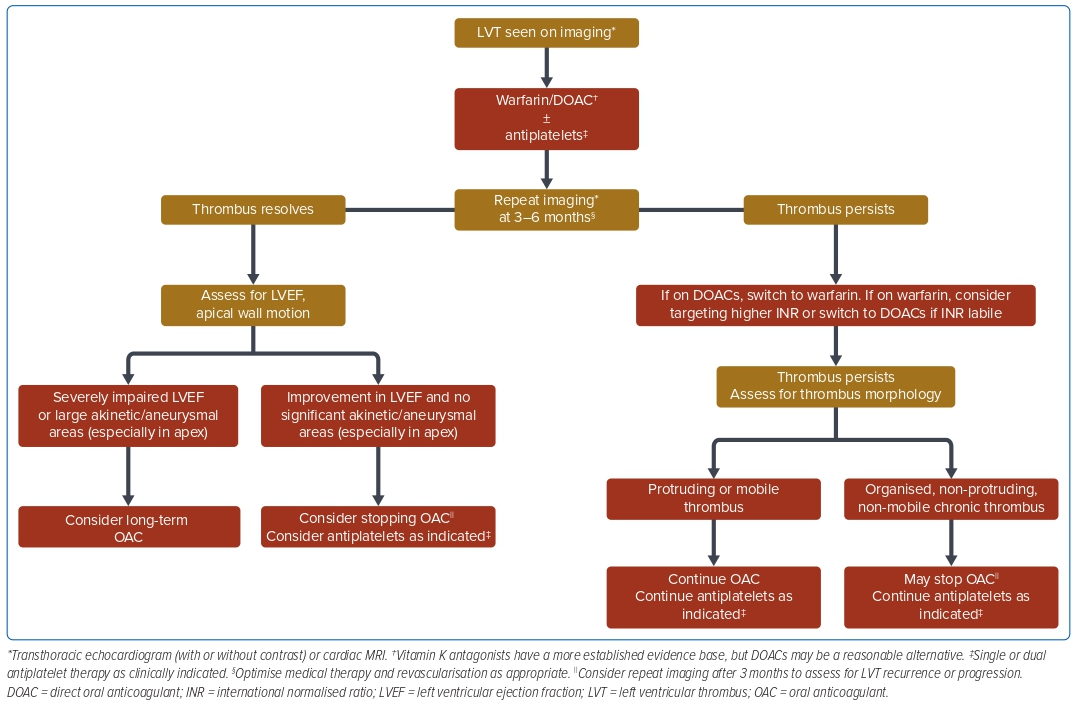
Synthesising the available published evidence and literature, in Figure 2 we propose a simple, practical approach to the management of LVT. Patients with risk factors for LVT should be imaged initially with TTE with or without contrast, with consideration of CMR if clinical suspicion is high and TTE is negative.53 The choice of initial anticoagulation for most patients would be warfarin due to the larger evidence base, however, DOACs maybe a reasonable alternative given the growing literature supporting its use. In all cases it is important to continually optimise medical management and perform revascularisation, as clinically appropriate. TTE and/or CMR should be repeated at 3–6 months to assess for thrombus resolution.
In patients with persistence of thrombus, if they were initially on DOACs, switching to warfarin can be considered. For patients on warfarin, aiming for a higher target INR (e.g. increasing the target INR range from 2–3 to 2.5–3.5) can be considered. If the thrombus continues to persist on follow-up TTE, an assessment for the morphology of thrombus on TTE may be useful to guide further management. In patients who have high-risk features for embolisation (e.g. mobile or protruding thrombus), continuation of OAC should be considered, taking into account the bleeding risk. In patients with a mural, organised or calcified thrombus without high-risk features, withdrawal of OAC may be considered.
In patients with thrombus resolution, the LVEF and apical wall motion may facilitate decision-making with regard to the continuation of anticoagulation. In a patient with persistently depressed LVEF or large akinetic/aneurysmal areas of the LV, especially in the apex, continuation of OAC should be considered taking into account the bleeding risk. In patients with a significant improvement in LVEF and improved wall motion, OAC may be stopped and antiplatelet therapy continued as per clinical indications. In either case, upon cessation of anticoagulation, follow-up TTE should be performed to assess for thrombus recurrence.
Limitations
The conclusions derived from this review are subject to several limitations. Reflecting the current evidence base, the vast majority of studies included in this review were observational studies, which are inherently more prone to bias.56 Only three RCTs were available for inclusion, which were open-label in nature (owing to the use of warfarin and need for INR monitoring) and enrolled a relatively small number of participants, attributable to the difficulty in recruiting and randomising patients with LVT, which is not particularly common. The practical algorithm proposed was based on the synthesis of the available limited literature and expert opinion and will need to be taken in context in the management of the individual patient.
Conclusion
Current evidence on the management of LVT is limited. This updated review summarises the available evidence for the management for LVT and proposes a practical algorithm for the management of LVT. Future adequately powered RCTs with longer follow-ups are needed to further validate these recommendations.
Click here to view Supplementary Material.
Clinical Perspective
- With regards to the initial choice of anticoagulation for left ventricular thrombus (LVT), although warfarin has a larger evidence base, several studies have suggested that direct oral anticoagulants are just as effective and have a better safety profile.
- In patients with persistent LVT, aiming for a higher target international normalised ratio or switching to a different class of anticoagulants may aid in clot resolution.
- In patients with LVT resolution, certain high-risk features for recurrence, such as persistently depressed LV ejection fraction or apical wall akinesia, may prompt longer-term anticoagulation after discussion with the patient regarding the risks.