Severely calcified lesions have a negative impact on the procedural and clinical outcomes of percutaneous coronary intervention (PCI).1,2 Heavily calcified lesions impede adequate stent expansion, resulting in increased intraprocedural complications and unsatisfactory long-term outcomes.2 Atherectomy techniques are effective in achieving acute luminal gain but are associated with periprocedural complications, while specialty balloons are limited in debulking effect.3,4 Intravascular lithotripsy (IVL) has gained considerable interest due to its unique mechanism of modifying superficial and deep wall calcification, as well as its operatorfriendliness and intuitive learning curve.5 Recently, IVL therapy has demonstrated reasonably good long-term efficacy and safety in large allcomers IVL registries and prospective trials.6,7 However, several high-risk patient groups were excluded from the prospective DISRUPT-CAD IVL safety and efficacy trial series.6 For example, the DISRUPT CAD trials excluded patients with creatinine >2.5 mg/dl or dialysis, patients with recent MI within 30 days or left-sided heart failure, poorly controlled diabetes, and a high SYNTAX score >33.6,8 These groups of patients, however, represent a real-world cohort in which renal failure, complex multivessel, or left main disease also commonly coexist.9–12 In these widely encountered, complex patient cohorts, the long-term safety and efficacy profiles of IVL remain uncertain.
In left main disease, optimal stent expansion is a significant indicator of long-term outcome.13–15 Calcified disease in the left main artery is associated with stent underexpansion and increased re-stenosis risk.16 Considering the large area of myocardium subtended by the left main artery, lesion preparation by debulking with atherectomy and cutting balloons carries heightened risk, including perforation, dissection and noreflow.17 As such, IVL offers a reasonable adjunct to treat calcified left mains, although it involves the challenge of requiring prolonged balloon inflation. Given these considerations, short- and long-term outcomes of calcium modification with IVL therapy are of interest but are presently lacking.
During PCI, underlying heavy coronary calcifications often impede optimal stent expansion during implantation.16 Inadequate expansion is associated with increased risk of stent thrombosis and compromises long-term luminal patency, resulting in future re-stenosis.18 When encountering stent underexpansion during PCI, treatment options are often limited. Rotational atherectomy use may risk stent damage, no-reflow and burr entrapment, while aggressive repeated post-dilatation with non-compliant balloons carries a risk of perforation.13,19 IVL may be helpful in this setting due to its ability to crack the deeper layers of calcium behind the stent.14 However, IVL use for stent underexpansion remains off-label and excluded from prospective trials.15
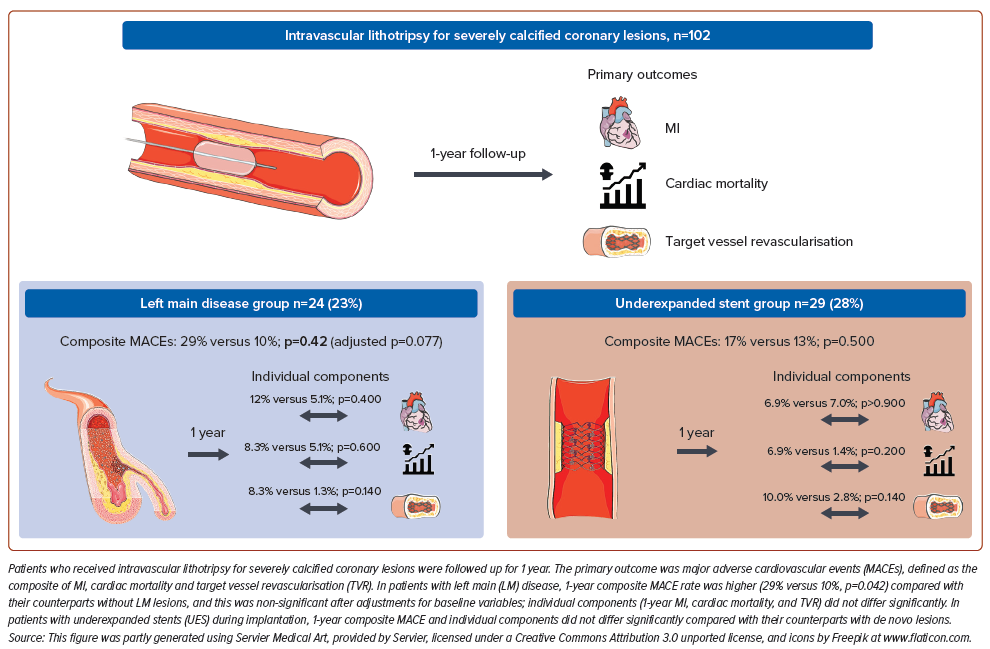
In this paper, we sought to examine the 1-year clinical outcomes of our IVL all-comers cohort, with particular emphasis on two subgroups, namely left main coronary artery (LMCA) stenosis, and stent underexpansion due to underlying severe coronary calcifications.
Methods
Study Design
We performed a longitudinal observational study of all patients who received IVL for severely calcified coronary lesions at our tertiary centre from January 2020 to December 2021 and followed them for 1 year or until death. Baseline demographic and clinical data were obtained from the national databank of cardiovascular diseases, and procedures used for national audit, quality improvement and research purposes.20 Inhospital and 30-day outcomes of our cohort have been previously described.1 Here, we describe the 1-year major adverse cardiovascular events (MACEs), defined as a composite of all-cause mortality, MI and target vessel revascularisation (TVR) for all participants treated with IVL. In addition, prespecified analyses were performed for subgroups of interest, namely: participants with left main disease, defined as LMCA stenosis ≥50% (the left main [LM] group); and those with underexpanded stents (UES) due to heavy coronary calcifications (the UES group), defined as a restriction of >50% of the reference vessel diameter in a stented segment in which an appropriately sized non-compliant balloon inflated at high pressure failed to expand during stent implantation. This subgroup represents a population of patients in whom initial inflation of the stent balloon resulted in stent underexpansion, which required IVL to optimise stent apposition. Participants with neither LM disease nor UES were grouped as ‘Neither’.
Participants
Local regulations required participants to fulfil prespecified criteria to be eligible for IVL therapy: severe calcification on intravascular imaging, defined as a ≥270° arc of calcium; or angiographic evidence of severe calcifications where devices cannot cross, or a non-compliant balloon cannot adequately expand. For elective cases, additional consent for IVL use was required. IVL without consent was permitted as a bail-out in urgent cases after initial lesion modification attempts had failed. Local regulatory directives required all IVL outcomes data to be collected for healthcare audits; hence, all cases of IVL from our institution were included. Informed consent for data collection was waived with permission from the institutional Centralised Institutional Review Board. This study was carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Study Device and Procedures
The IVL catheter (shock wave C2 , Shock Wave Medical) contains an integrated balloon with lithotripsy emitters that generate sonic pressure waves. As per the standard technique, the balloon catheter was sized 1:1 to the reference artery and deployed to the target lesion. It is inflated to low pressures and delivers up to 10 pulses per cycle (1 pulse/second over 10 seconds). Adjunctive tools, such as buddy wire, a small balloon, or guidewire extensions, were allowed if the operator could not pass the IVL catheter across the lesion. In the event of multiple lesions, each lesion was treated with a minimum of 20 pulses. If lesion preparation was incomplete, other IVL catheters with different balloon sizes were allowed. Pre- and post-dilatation was permitted at the individual operator’s discretion.
Endpoints
The primary endpoints were 1-year MACE rate, defined as a composite of MI, TVR or cardiac mortality. The secondary endpoints were periprocedural complications, including perforation and slow or no reflow, length of stay, all-cause mortality, stroke, and in-hospital and 30-day MACEs. MI was defined as per the fourth universal definition of MI.21
Statistical Analysis
Continuous data are given as median (IQR), and categorical data are presented as counts and proportions (%). For the comparison of groups, Person’s Χ2 test and Fisher’s exact test were used for categorical variables, and the Mann–Whitney U-test was used for continuous variables. Twosided p≤0.05 was considered statistically significant. Advanced chronic kidney disease (CKD) was defined as estimated glomerular filtration rate (eGFR) ≤30 ml/min/1.73 m2 or being on chronic dialysis. Logistic regression was performed on 1-year MACEs, and additional multivariable regression was performed using variables with p<0.100 on univariable analysis. Statistical analysis was performed using R software version 4.1.2 (R Foundation).
Results
Participants and Procedures
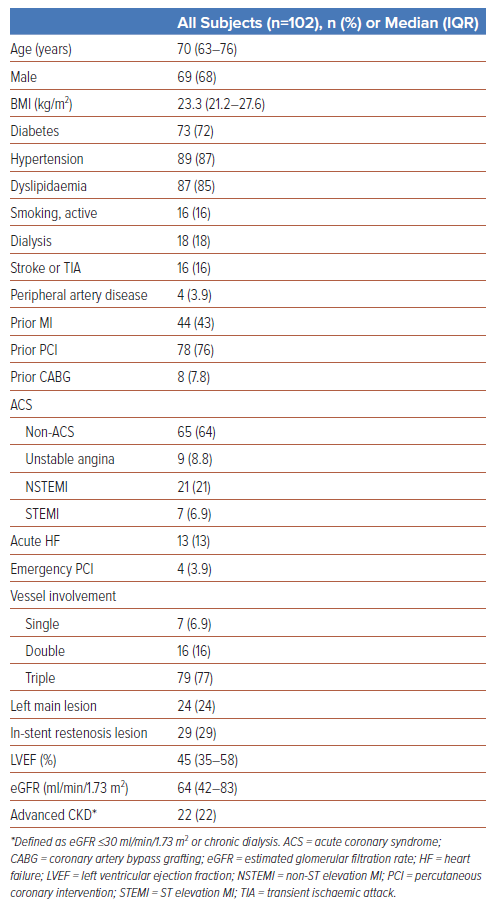
One hundred and two participants were studied (median age, 70 years; 68% male; Table 1). The baseline demographics have been previously described.1 Of note, there was a high prevalence of cardiovascular risk factors, with 72% of participants having diabetes, 87% having hypertension, 85% having dyslipidaemia, 16% actively smoking, and 22% with advanced CKD. A considerable proportion of participants had established cardiovascular disease, including 84% with prior coronary artery bypass grafting (CABG) or PCI and 43% with previous MI.
Nearly half presented acutely with either acute coronary syndrome (ACS; 36%) or heart failure (13%). Almost all participants had multivessel disease (93%), including 77% with triple-vessel disease and 24% with LM disease. Median left ventricular ejection fraction (LVEF) was reduced (45%, IQR 35–58%).
There were 24 participants in the LM group (23.3%) and 29 in the UES group (28.4%). Supplementary Figure 1 shows the detailed breakdown of participants between the two subgroups. Six participants (5.9%) had both LM disease and UES. Fifty-five participants (53.9%, Neither group) had neither LM disease nor UES.
Participants in the prespecified subgroups (LM and UES) had a higher cardiovascular disease burden than their counterparts (versus non-LM and de novo lesions, respectively). Participants in the LM group were more likely to be older (74 years versus 68 years, p=0.034), have diabetes (88% versus 67%, p=0.048), hypertension (100% versus 83%, p=0.035), advanced CKD (42% versus 17%, p=0.010), and reduced LVEF (39% versus 50%, p=0.010) than participants without LM lesions (Supplementary Table 1). Participants in the UES group were more likely to have advanced CKD (38% versus 15%, p=0.014), prior MI (62% versus 37%, p=0.020), and prior PCI (100% versus 68%, p<0.001) than those with de novo lesions (Supplementary Table 2).
Outcomes
Overall Cohort Outcomes
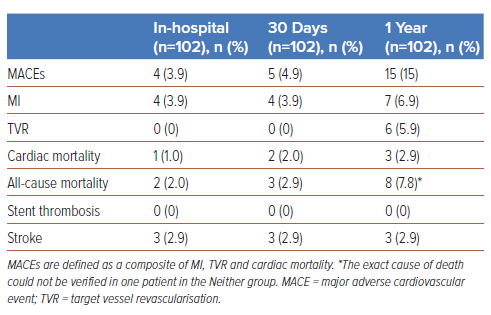
The overall periprocedural complications and in-hospital and 30-day MACE rates of our IVL all-comers cohort have been previously described.1 Between the 30-day and 1-year timepoints, three additional cases of MI, one cardiac mortality, and six TVRs occurred, resulting in an overall 1-year MACE rate of 15% (MI 6.9%, cardiac mortality 2.9%, TVR 5.9%; Table 2, Figure 1). One year overall all-cause mortality was 7.8% and stroke was 2.9%. All incidences of stroke occurred during in-hospital stays, and there were no other cases subsequently up to 1 year. There were no incidences of stent thrombosis in the duration of this study.
Left Main Disease
Primary Endpoints
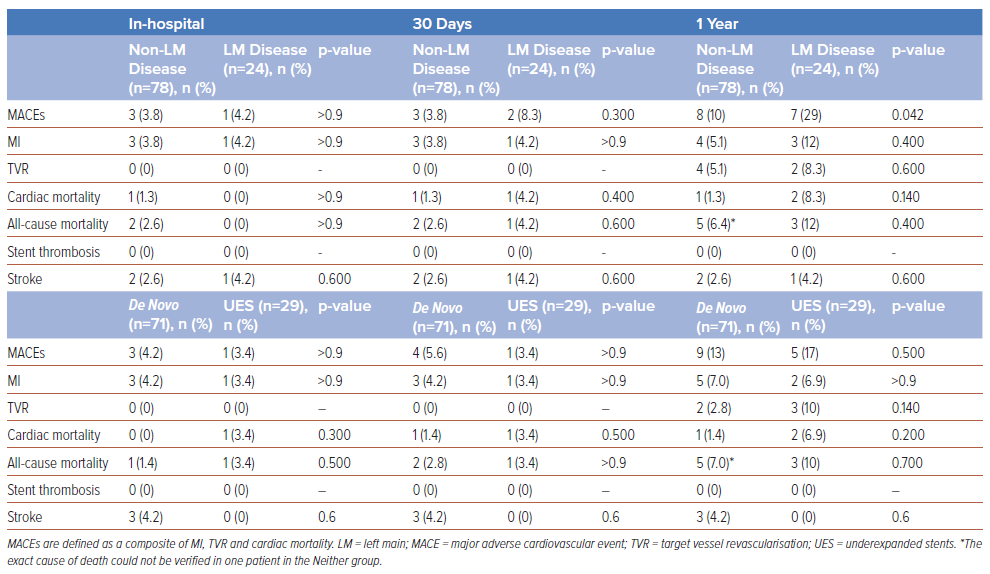
Rates of in-hospital (4.2% versus 3.8%, p>0.900) and 30-day MACEs (8.3% versus 3.8%, p=0.300) were not significantly different between the LM group and the participants without LM disease (Table 3). At 1 year, the MACE rate was significantly higher in the LM group than in the non-LM group (29% versus 10%, p=0.042). Individually, the rates of 1-year MI (inhospital 4.2% versus 3.8%, p>0.900; 30-day 4.2% versus 3.8%, p>0.900; 1-year 12% versus 5.1%, p=0.400), cardiac mortality (0 versus 1.3%, p>0.900; 4.2% versus 1.3%, p=0.400; 8.3% versus 1.3%, p=0.140) and TVR (1-year 8.3% versus 5.1%, p=0.600) did not differ significantly between the LM and non-LM groups (Table 3). Univariable logistic regression predicting 1-year MACEs showed that the presence of significant LM disease (OR 3.60; 95% CI [1.12–11.5]; p=0.028) and non-ST elevation MI (OR 3.93; 95% CI [1.09–14.3]; p=0.034) were associated with increased MACE risk after treatment with IVL (Supplementary Table S3). Further analysis with both multivariable regression (OR 3.08; 95% CI [0.87–10.9]; p=0.077) and propensity score-adjusted regression (OR 3.77; 95% CI [0.95–15.7]; p=0.060) found that LM was no longer predictive of 1-year MACEs after adjustment for covariates, including ACS presentation and contrast volume.
Secondary Endpoints
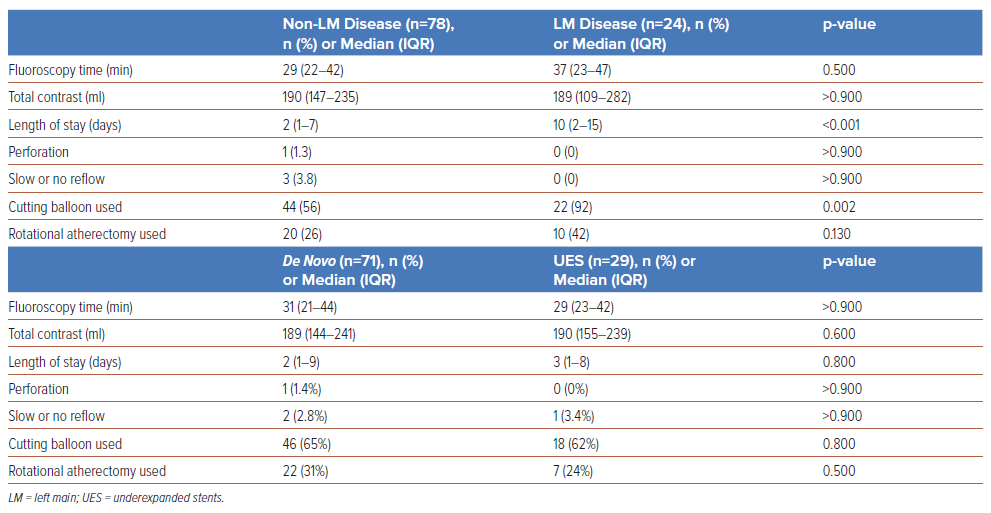
A greater proportion of participants in the LM group required cutting balloon usage (92% versus 56%, p=0.002) and the median length of stay was longer (10 days versus 2 days, p=<0.001) in comparison with the nonLM group (Table 4). There were no instances of perforation (0 versus 1.3%, p>0.900) or slow-/no-reflow (0 versus 3.8%, p>0.900) in the LM group, compared with the non-LM participants. One-year all-cause mortality (12% versus 6.4%, p=0.400) and stroke (4.2% versus 2.6%, p=0.600) were not significantly different between the groups (Table 3). There were no occurrences of TVR at 30-day follow-up; 1-year TVR was not significantly different between the LM group and non-LM participants (8.3% versus 5.1%, p=0.600)
Stent Underexpansion
Primary Endpoints
In-hospital (3.4% versus 4.2%, p>0.900) and 30-day (3.4% versus 5.6%, p>0.900) MACEs were not significantly different between the UES group and the participants with de novo disease. At 1 year, MACE rates were not significantly different between the UES group and the de novo lesions group (17% versus 8.5%, p=0.300; Table 3).
Secondary Endpoints
There were no significant differences in perforation (0 versus 3.4%, p>0.900) or slow-/no-reflow (3.4% versus 2.8%, p>0.900) between the UES group and the de novo lesions group. There were no occurrences of TVR at 30-days follow-up; 1-year TVR was not significantly different between the UES group and participants with de novo lesions (10% versus 2.8%, p=0.140).
Discussion
To our knowledge, this is the first study on the long-term safety of IVL therapy in a high-risk real-world population. In this study, more than twothirds of participants had prior revascularisation (CABG or PCI), and almost half presented with ACS or decompensated heart failure; nearly all participants had multivessel coronary artery disease, and the median LVEF was 45%. Furthermore, participants in our prespecified subgroups had an even higher cardiovascular burden than their counterparts. Overall, our data suggest that with IVL therapy, even patients with more complex anatomy and multiple comorbidities can achieve acceptable cardiac outcomes. Our study findings highlight that these patients with multiple comorbidities, often underrepresented in clinical trials, form a significant proportion of cases presenting to the catheterisation lab with calcific disease. The inclusion of participants with advanced CKD, LM disease, ACS and acute heart failure contributed to differences in 1-year clinical outcomes as compared with DISRUPT CAD III (all-cause mortality, 7.8% versus 1.8%; cardiac mortality, 2.9% versus 1.1%). Despite these limitations, we found comparable rates of MACEs (15% versus 13.8%), MI (6.9% versus 10.5%), and TVR (5.9% versus 6.0%) with the DISRUPT CAD III cohort at 1 year.
In participants with LM disease, MI, stroke and TVR the rates did not differ significantly from those without LM. However, the overall composite MACE rate was considerably higher in participants with LM (29% versus 10%). There were higher rates of cardiac mortality (8.3% versus 1.3%) and all-cause mortality (12% versus 6.4%) compared with the non-LM group at 1 year, although this was not statistically significant. Logistic regression with covariate adjustments (using ACS presentation and contrast volume) found that LM was no longer significantly associated with 1-year MACE rate, implying that the difference in MACE rate between LM and non-LM participants receiving IVL can possibly be attributed to the higher prevalence of comorbidities as opposed to the effect of IVL. For these reasons, the 1-year MACE rate of our LM IVL cohort is higher compared with contemporary randomised studies of PCI in LM disease (i.e. EXCEL and NOBLE trials).22,23 Supporting this, the LM group’s mean length of stay was 10 days compared with 2 days in the non-LM group (p<0.001). The higher burden of higher multimorbidity, including older age, diabetes, advanced CKD and poorer LVEF, were likely to have conferred poorer physiological reserves and, when compounded by the higher complexity of LM PCI, presumably translated to more extended inpatient care requirements and length of stay. Furthermore, long-term outcomes of IVL therapy for LM stenosis in other studies are also lacking. The DISRUPT studies excluded participants with unprotected LM stenosis diameter >30%, ostial lesions of the left anterior descending or circumflex arteries, and bifurcation lesions with stenosis >30%.6 Similarly, LM participants were excluded from (ROTAXUS) or underrepresented in (ORBIT II) the atherectomy studies.24,25 Of note, there were no instances of perforation, or slow or no reflow in the LM group, further supporting the safety profile of IVL in LM PCI. We hope that the findings from our research help fill this gap and generate further interest regarding the optimal strategy to treat calcific LM disease.
In the setting of stent underexpansion during implantation, heavy underlying calcifications often impede adequate stent apposition and expansion. Using IVL in such a scenario enables cracking of deeper layers of calcium beneath the stent struts without significant risk of stent damage. This study did not observe a significant difference in MACE rate at 1 year after IVL therapy for UES compared with their counterparts. Long-term outcomes of IVL therapy for UES are also lacking. Like the LM subgroup, patients with UES were excluded from the DISRUPT CAD, ROTAXUS and ORBIT II studies and this remained an off-label indication for IVL use.6,15,24,25 The multicentre IVL-DRAGON registry previously reported on 30-day outcomes of IVL therapy for stent underexpansion.14 There were fewer participants with diabetes (45.2% versus 83%) and on dialysis (1.6% versus 38%) in IVL-DRAGON; cardiac mortality occurred in one participant (1.6%), and there was no TVR or MI at the 30-day follow-up.
In our UES cohort, one MI (3.4%) and one cardiac mortality (3.4%) occurred in hospital with no additional cases at 30 days, similar to the short-term findings for IVL therapy in IVL-DRAGON. In addition, the incidences of perforation (0%) and slow or no reflow (n=1, 3.4%) were low in the UES group, highlighting the procedural safety of IVL therapy for stent underexpansion. We hope our results will highlight IVL as a safe therapy for stent underexpansion due to coronary calcium.
The additional utility of IVL therapy lies in its unique ability to crack the intimal and deeper medial vessel wall calcifications that are frequently observed in patients with advanced CKD, especially when eGFR ≤30 ml/ min/1.73 m2 , or in patients on chronic dialysis.8,26,27 Advanced kidney disease is often associated with heavy coronary calcifications that impede percutaneous coronary revascularisation, leading to unfavourable angiographic and clinical outcomes at short- and long-term follow-up.8,26 Autopsy studies of patients with advanced kidney disease have demonstrated severe calcifications due to amorphous calcium and phosphorus accumulation involving both the intimal and deeper medial vessel walls, which are absent in milder forms of renal disease.27 Deepseated calcifications increase wall stiffness and limit stent expansion; thus, luminal gain by intimal debulking alone may not be sufficient.28,29
Despite the significantly higher prevalence of advanced CKD in both the LM and UES groups, we observed that the rates of TVR at 1 year were low and comparable to non-LM participants and participants with de novo lesions. This indirectly suggests that IVL therapy provides reasonable long-term outcomes even in the presence of advanced kidney disease or in patients on chronic dialysis, who often have extensive calcific atherosclerotic burden involving both the intimal and medial vessel wall layers. This hypothesis that IVL may help improve PCI outcomes in advanced CKD patients warrants further clinical studies.28 Given the limited existing data presently available, the data from our small cohort support IVL as an acceptable adjunctive therapy for the treatment of calcified coronary lesions in patients with advanced or end-stage kidney disease.
Limitations
Several significant limitations should be considered when interpreting the results of our study. First, this retrospective single-centre study may introduce biases and limit generalisability. The open-label nature of this registry study may introduce selection bias and is arguably dependent on operator experience. Nevertheless, given the lack of long-term data on these high-risk cohorts, these findings help guide subsequent larger prospective longitudinal studies to fill this knowledge gap. Second, intracoronary imaging was not used in a large majority of cases. Third, the study period started when IVL therapy was considered relatively new; increasing operator experience and patient or lesion selection is likely to reduce adverse outcomes. Fourth, comparisons with other lesion modification techniques, such as atherectomy or specialty balloons, lack head-to-head comparisons and are hypothesis-generating at best. Fifth, the sample sizes of the prespecified LM and UES subgroups were small, making statistical analysis and interpretation challenging. Last, this study does not provide information about hybrid strategies such as RotaTripsy; future studies in these directions are warranted and encouraged.
Conclusion
Our findings provide important long-term clinical data on IVL therapy in real-world all-comers and two complex cohorts. Long-term adverse event rates were higher in LM disease, probably due to the significant concomitant comorbid burden. Overall, our data support the addition of IVL to the growing armamentarium for calcium modification in LM disease and stent underexpansion during implantation due to severe coronary calcifications. Larger prospective trials and head-to-head comparisons with other established plaque-modification therapies are warranted for definitive conclusions.
Clinical Perspective
- Data on long-term outcomes of intravascular lithotripsy (IVL) in high-risk populations are lacking.
- Based on our 1-year clinical outcomes data in all-comers with severely calcified coronary arteries, major adverse cardiovascular events (MACEs) and the components (MI, cardiovascular death, or target vessel revascularisation) did not differ significantly in participants with stent underexpansion during implantation due to severe coronary calcifications, compared with de novo lesions.
- MACE rate was higher in left main disease and this was likely to be due to the elevated cardiovascular comorbidity burden and acute coronary syndrome presentation.
- Our data support the addition of IVL to the growing armamentarium of calcium modification techniques.
- However, larger prospective trials and head-to-head comparisons with other established plaque-modification therapies are warranted for definitive conclusions.