Atrial septal defects (ASD) are an uncommon cause of right heart failure and pulmonary hypertension. Sinus venosus ASD (SVASD) are an uncommon form of ASD, amounting to 5–10% of all ASD. They are commonly underdiagnosed as the defects are often located superior and adjacent to the superior vena cava (SVC), which is often visualised poorly on conventional transthoracic echocardiography (TTE).1 Reported here is a case of delayed presentation of SVASD in an elderly patient, with clinical features mimicking that of liver cirrhosis, diagnosed following agitated saline test and multimodality imaging.
Case Report
A 59-year-old man was referred to the cardiology unit for assistance in managing newly diagnosed AF. He was under the routine care of the gastroenterology team for thrombocytopaenia, with potential liver cirrhosis. However, investigations including oesophagastroduodenoscopy, colonoscopy, ultrasound imaging of the hepatobiliary system and endoscopic ultrasonography of the liver were unremarkable for any abnormalities. He had been complaining of worsening dyspnoea and reduced effort tolerance over the past 6 months. His vital signs in the clinic included a blood pressure of 128/65 mmHg, pulse rate of 94 BPM, respiratory rate of 16 breaths/minute, oxygen saturations of 95% on room air and temperature of 36.4°C. Clinical examination was otherwise unremarkable, aside from an irregularly irregular pulse. The initial ECG revealed absence of P waves, with an irregularly irregular rhythm supportive of AF. In addition, there was evidence of right axis deviation, prominent R waves in lead V1-2 and prominent S waves in leads V3–V6 (Supplementary Figure 1). Chest radiography demonstrated cardiomegaly with a rounded left heart border.
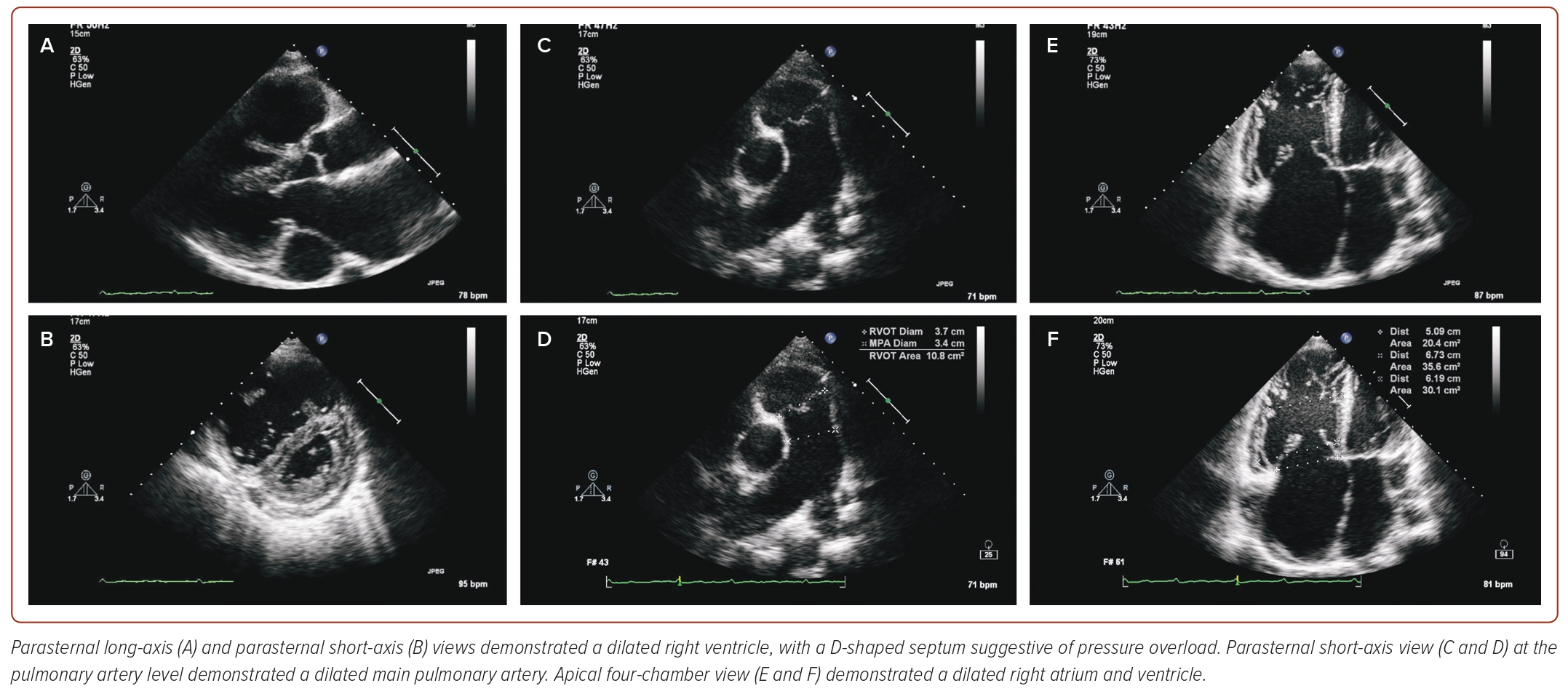
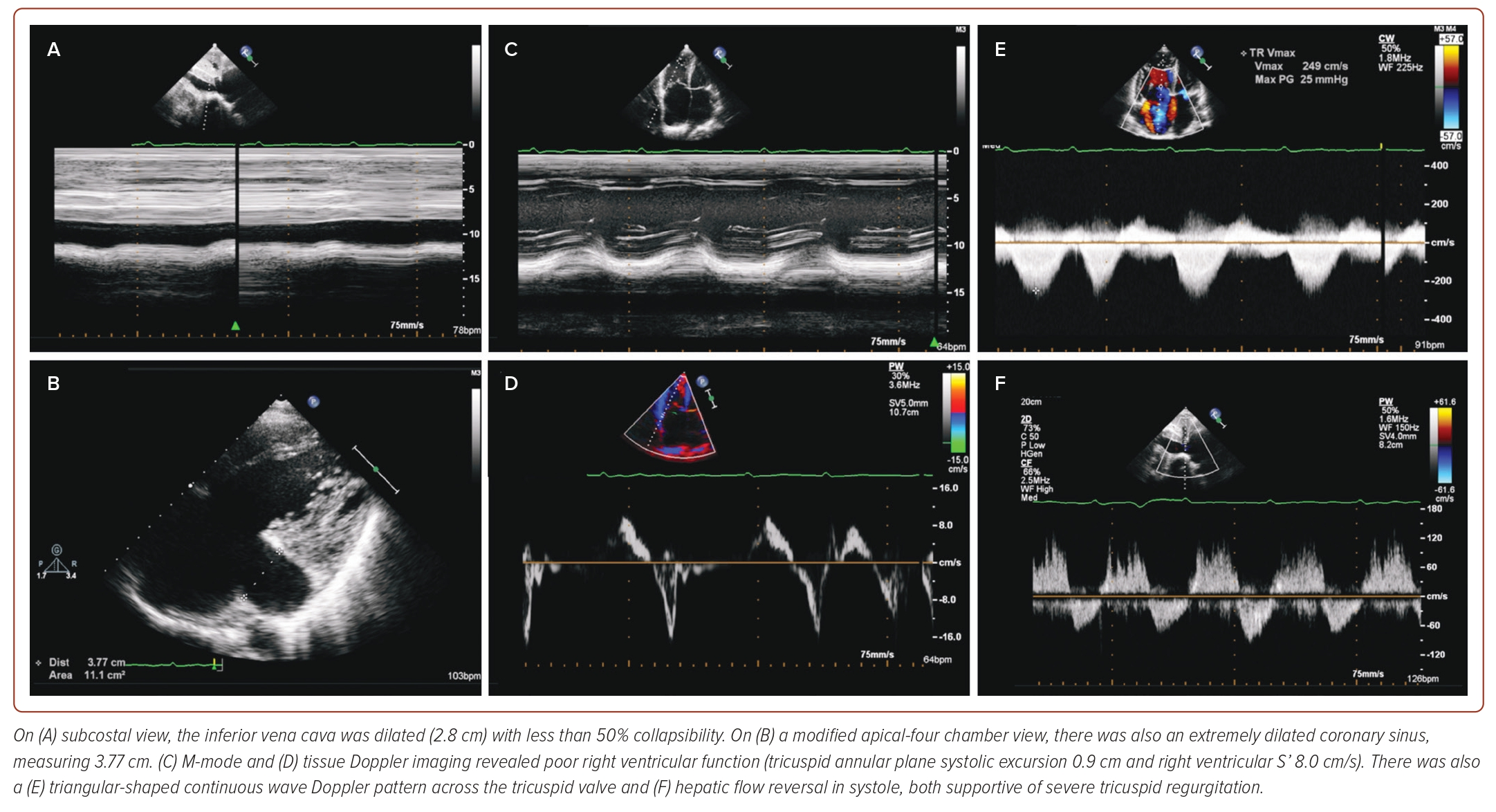
A TTE was performed as part of the patient’s initial work-up, demonstrating both a dilated right atrium (right atrial volume 48 ml/m2) and right ventricle (basal diameter 6.73 cm and mid diameter 6.19 cm), with relatively normal sized left atrium and ventricle (Figure 1 and Supplementary Video 1). There was also evidence of a D-shaped septum during both systole and diastole, highly suggestive of pressure overload and a dilated main pulmonary artery. The tricuspid valve (TV) leaflets showed poor coaptation, leading to torrential tricuspid regurgitation (TRl) (Supplementary Video 2). The inferior vena cava was dilated (2.8 cm) with less than 50% collapsibility, with an extremely dilated coronary sinus measuring 3.77 cm. There was evidence of poor right ventricular function (tricuspid annular plane systolic excursion of 0.9 cm and right ventricular S’ of 8.0 cm/s), alongside a triangular-shaped continuous wave Doppler pattern across the TV and hepatic flow reversal in systole, supportive of severe TR and suggestive of severe pulmonary hypertension (Figure 2). However, close interrogation of the interatrial septum using standard TTE views did not reveal the presence of any defects to explain the findings on TTE.
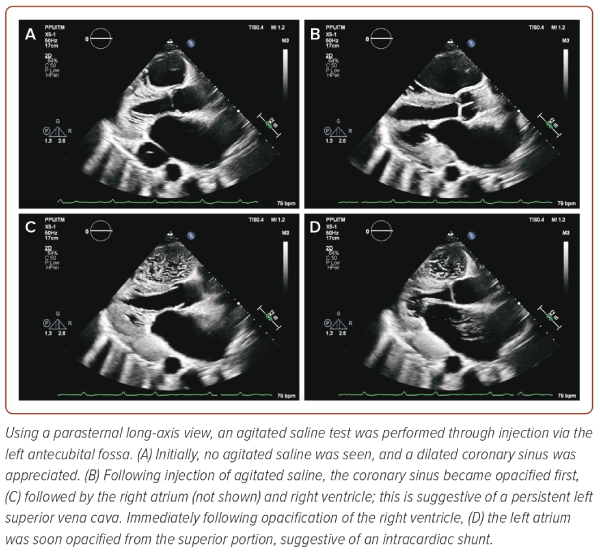
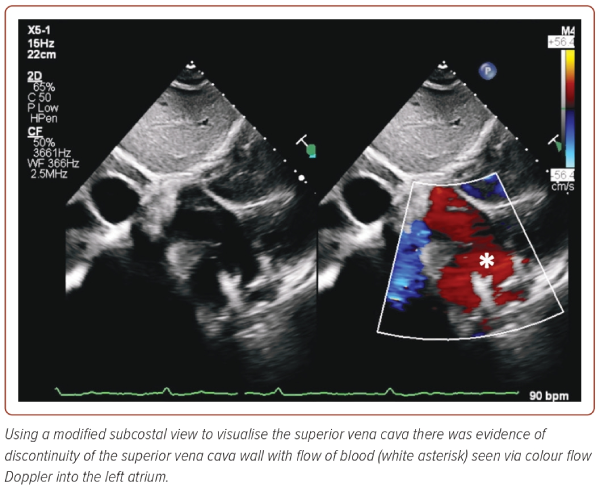
Following the discovery of the enlarged coronary sinus, agitated saline was injected through an IV access located in the left antecubital fossa, in anticipation of a persistent left SVC (PLSVC) (Figure 3 and Supplementary Video 3). Indeed, injection through the left fossa resulted in opacification of the coronary sinus first, followed by the right atrium (not shown) and ventricle suggestive of a PLSVC. In addition, agitated saline was seen travelling into the superior portion of the left atrium as well within a cardiac cycle of opacification of the right ventricle, raising suspicion of an intracardiac shunt. Furthermore, on non-conventional subcostal views on TTE, discontinuity along the walls on the SVC was visualised as well, raising the suspicion of an SVC defect although its exact contribution to the intracardiac shunting remained poorly understood (Figure 4).
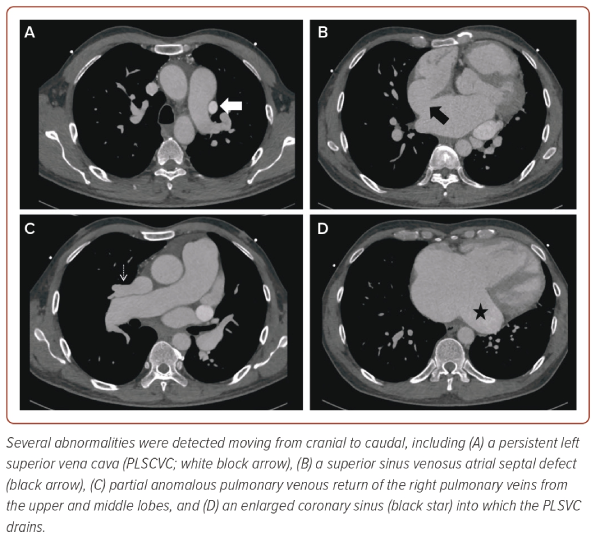
The patient was thus scheduled for CT imaging of the thorax, revealing a superior SVASD alongside the PLSVC demonstrated on TTE (Figure 5 and Supplementary Video 4). There was also evidence of an anomalous venous drainage of the pulmonary veins from the right upper and middle lobes, draining into the right SVC and subsequently into the right atrium. Invasive coronary angiography demonstrated absence of any coronary artery disease. Following a multidisciplinary discussion, the patient was referred to the nearest adult congenital heart disease service team for consideration of surgical closure, which he underwent successfully and remains under close surveillance.
Discussion
We have described a unique case of delayed diagnosis of symptomatic SVASD in a patient presenting in his sixth decade of life. SVASD are considerably uncommon compared to other types of ASD and remain generally undiagnosed until the fifth decade of life following the development of symptoms linked to right heart failure or pulmonary hypertension.2
SVASD occur as a result of incomplete or absence of septation between the pulmonary vein and superior vena cava or right atrium and are commonly associated with partial anomalous pulmonary venous return (PAPVR) as seen in the present case.3,4 In fact, up to 90% of SVASD are associated with PAPVR, which exacerbates the left-to-right shunting that occurs and increases predisposition to Eisenmenger physiology.4 Complications associated with SVASD include pulmonary hypertension, arrhythmias and extrinsic compression of the pulmonary artery.1,5
TTE has poor sensitivity (about 10%) in diagnosing SVASD, highlighting the importance of multimodality imaging in such cases.3,6 Furthermore, multimodality imaging allows for detection of associated congenital anomalies, both common ones like PAPVR, and somewhat rarer associations like PLSVC, as seen in the present case.2,6,7 Few case reports have been published on co-existing SVASD and PLSVC.6,8,9 The majority of PLSVC (92%) drain into the right atrium, via the coronary sinus, which further contributes to right ventricular volume overload.8,9 Diagnosing a PLSVC is important to avoid complications following cannulation and instrumentation of vascular structures perioperatively, and prior to insertion of cardiac implantable electronic devices, which have been reported.6,9 The present case is unique as it highlights how interrogation on TTE using non-conventional modified views alongside an agitated saline test proved pivotal in making the diagnosis of an SVASD, and serves as a reminder that a dilated coronary sinus often requires thorough evaluation. Despite the poor sensitivity of TTE in diagnosing SVASD, features suggestive of a significant intracardiac shunt are far easier to appreciate on TTE. Therefore, lack of structural ‘drop out’ within the interatrial septum should not deter clinicians from performing additional investigations including agitated saline test or other forms of cardiac imaging to help with obtaining a right diagnosis.
Conclusion
SVASD remains an uncommon, but important, diagnosis to recognise as patients can often be misdiagnosed, leading to mismanagement of the condition. The purpose of this case report is to highlight the unique features demonstrated on TTE, especially during the agitated saline study, which showcased the presence of an PLSVC alongside an intracardiac shunt which was undetected through conventional 2D TTE views, requiring modified angulations. Nevertheless, as highlighted, TTE is often inadequate, and both a high index of suspicion and multimodality imaging are paramount in securing a diagnosis.