Aortic stenosis is a valvular heart disorder often seen in older people which causes left ventricular obstruction, causing symptoms such as syncope, angina, dyspnoea and heart failure symptoms. Common aetiologies include congenital valve problems, such as unicuspid or bicuspid valve, calcific (formerly called degenerative) and rheumatic disease. Rheumatic disease is the most frequent acquired cause of aortic stenosis and in developing countries, aortic stenosis is not treated by medical management alone.1
Definitive treatment of severe aortic stenosis is aortic valve replacement (AVR), either using an open surgical (SAVR) or transcatheter (TAVR) approach. In the American Heart Association/American College of Cardiology (AHA/ACC) 2021 guidelines, SAVR is the recommended approach for patients over 65 years of age or those who are expected to live for more than 20 years after treatment. For patients aged 65–80 years old with no anatomical contraindication for TAVR, either SAVR or TAVR is recommended and the choice is based on shared decision-making between care provider and patient.2 In terms of valve choice for replacement, latest guidelines recommend the use of mechanical AVR for patients under 50 years old and a bioprosthetic valve for those over 65, owing to the possibility of degeneration of the bioprosthetic valve when used at an earlier age. In addition, the choice between mechanical valve or bioprosthetic valve should be based on a shared decision-making process between the doctor and the patient with consideration made to preference regarding the risks of anticoagulant therapy and potential valve reinterventions.2 Regardless of approach and type of valve, AVR is indicated even if symptoms are mild. After the procedure, most patients experience relief of symptoms of exertional dyspnoea and anginal episodes together with improved left ventricular ejection fraction.
Aortic valve surgery can lead to various complications, including paravalvular leaks, stent migration, arrhythmias, and stroke. Improperly fitted valves or deployment of the valve deep into the left ventricular outflow tract can compress the atrioventricular node or the His bundle, leading to conduction abnormalities. Calcific debris can also embolise into the cerebral circulation, potentially causing stroke.3
This case presents the possible role of rheumatoid arthritis (RA) in the development of early calcific aortic stenosis and will attempt to correlate the role of RA in predicting the risk for post-AVR complications of stroke and arrhythmias.
Case Presentation
The patient was a 44-year-old man from Guam who initially presented with shortness of breath at the emergency department in Guam. He was non-hypertensive and had diabetes. The patient had been diagnosed with RA 6 years ago, with a history of long-term steroid use for several months prior to this visit. He was taking three 5 mg prednisone tablets once a day. Previously, he was prescribed Golimumab for RA but later became non-compliant. He was adherent to therapy with golimumab, which is a tumour necrosis factor inhibitor and anti-inflammatory agent. However, the patient was lost to follow-up and had been non-adherent with medications for 2 years prior to this current admission in the Philippines. He had a family history of early cardiac death, hypertension and diabetes. The patient was a non-smoker but had a chronic addiction to chewing tobacco, although he rarely drank alcohol.
Seven months prior to his admission in the Philippines, he had experienced intermittent shortness of breath for 1 week, even while walking short distances, although this resolved with rest. This was accompanied by progressive bipedal oedema. The patient was admitted in Guam for 10 days and was managed as a case of heart failure with reduced ejection fraction in acute decompensation. Imaging diagnostics revealed a severe aortic stenosis; however, the patient was only treated medically due to lack of appropriate facilities for surgical intervention. He had shown a slight relief of symptoms with medical therapy alone.
One month prior to his present admission, additional work up was done in preparation for an elective AVR. Work up revealed abnormal myocardial perfusion scan, yet on coronary angiogram, revealed angiographically normal coronaries. Due to severe aortic stenosis alone, patient was advised to have minimally invasive SAVR via mini-thoracotomy.
A day prior to his elective surgery, patient had stable vital signs, tolerating low back rest (20–30 degree bed angle), with a noted grade 3/6 systolic murmur heard best at the base with no radiation to the apex and carotids. He had no bipedal oedema but was noted to have multiple joint deformities on both fingers – swan-neck deformities in the fingers, ulnar deviation of metacarpophalangeal joints and boutonniere deformity in both thumbs. A baseline neurological examination showed no focal deficits.
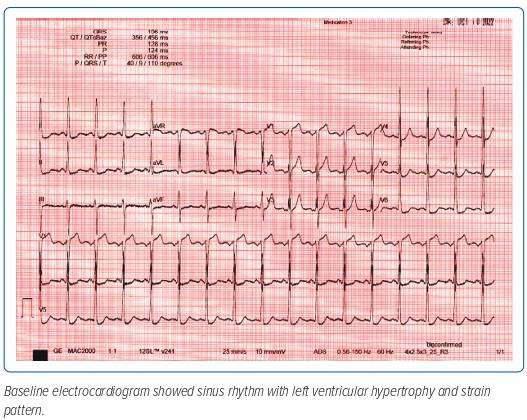
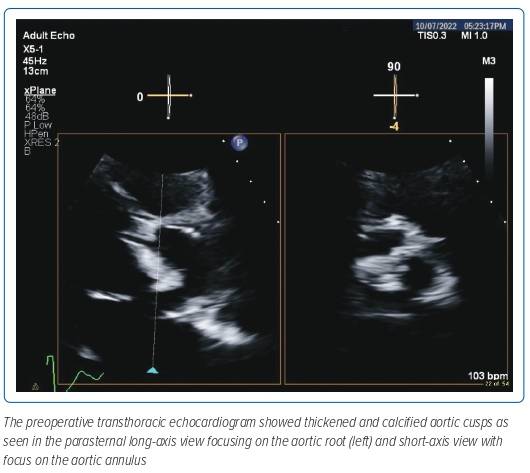
A preoperative baseline electrocardiogram showed normal sinus rhythm with left ventricular hypertrophy and strain pattern (Figure 1). Baseline 2D transthoracic echocardiogram (TTE) showed concentric left ventricular hypertrophy. Systolic function was severely reduced at 29.1% and there was severe global hypokinesia. The right ventricle was a normal size with normal systolic function and normal sized atria with no thrombus detected in the left atrial appendage which was functioning normally. There was an intact interatrial septum with lipomatous hypertrophy. The mitral valve leaflets were thickened with mild mitral regurgitation. The tricuspid valve was normal and had mild regurgitation. Severe calcific aortic stenosis was seen with an aortic valve area of 0.56 cm2 estimated by continuity equation and an indexed valve area of 0.4 cm2/m2 and a dimensionless velocity index of 0.12 with a mean gradient of 49 mmHg and peak velocity of 3.5 m/s (Figure 2). Moderate aortic regurgitation was also noted and the non-coronary cusp was noted to be immobile. Normal pulmonary pressures were noted.
The patient underwent Minimally invasive cardiac surgery SAVR using a bioprosthetic valve as per the patient’s preference, with a total ischaemic time of 163 minutes, bypass time of 231 minutes and a total operative time of 7 hours.
A postoperative 2D echocardiogram showed an improved ejection fraction from 29.1% to 34.5% with a note of a bioprosthetic valve well seated in the aortic position with an effective orifice area of 1.9 cm2 by continuity equation, velocity ratio of 0.31 with peak aortic velocity of 2 m/s, peak gradient of 17.4 mmHg and mean gradient of 8 mmHg. No rocking motion and no para-valvular leaks or transvalvular regurgitation was noted. The patient developed episodes of hypotension (after the patient was removed from bypass) and was started on a dobutamine drip (14 mg/kg/minute) and norepinephrine drip (0.06 mg/kg/minute). The patient was initially clinically stable after surgery but 2 hours later he was noted to have new-onset left-sided hemiparesis. A plain cranial CT scan was done which showed an acute frontal lobe infarct.
On the first postoperative day, the patient developed multiple episodes of ventricular tachycardia associated with hypotensive episodes, initially resolved by two defibrillations of 200 J, 150 mg amiodarone IV bolus or push and a magnesium sulphate bolus. Ventricular tachycardia converted to sinus tachycardia initially, but was later converted to multiple episodes of supraventricular tachycardia associated with hypotensive episodes and febrile episodes, with the highest temperature of 39°C, in which 15 synchronised cardioversions were given, which eventually converted the rhythm to sinus tachycardia.
The patient was eventually started on low dose carvedilol with an increased dose during the succeeding days in hospital. He was weaned off pressors and slowly weaned off the ventilator and he was eventually referred to the cardiac and physical rehabilitation programme, until he was discharged as well and stable. However, he had not been able to recover motor strength on the left extremities.
Discussion
Aortic stenosis has three primary causes: degenerative or calcific, congenital or acquired factors. Some common acquired causes include metabolic disorders, such as Fabry’s disease, irradiation and systemic lupus erythematosus, among others. While RA is a rheumatological condition that has been linked to aortic stenosis, its influence on the progression of the disease remains uncertain.
RA is a chronic autoimmune disorder that primarily affects women and the risk of developing the condition increases with age. RA typically affects synovial joints, starting with small peripheral joints and progressing gradually to proximal joints. Symptoms that persist for less than 6 months are classified as early RA, while those that last longer than 6 months are referred to as established RA. However, there is no definitive laboratory test for RA. Acute phase reactants like erythrocyte sedimentation rate and C-reactive protein are often elevated in patients with active disease but are not specific to this disorder. Signs of advanced disease include cartilage and bone erosion, periarticular osteopenia and narrowing of joint space.4
While RA is typically associated with musculoskeletal issues, it also has systemic effects that can affect the cardiovascular system. In RA patients, atherosclerosis can progress at a faster rate, leading to various atherosclerotic conditions such as ischaemic heart disease, MI and heart failure. This is partly due to the activation of the inflammatory cascade driven by tumour necrosis factor. However, the precise mechanisms underlying the impact of RA on the cardiovascular system are not yet fully understood.5
Several case reports and studies have explored the connection between RA and aortic stenosis. A study by Bois et al. found that patients with RA and a higher erythrocyte sedimentation rate had a slower rate of AS progression compared with the general population. A case report by Peyrou et al. described a patient with long-standing RA who progressed from moderate to severe low gradient AS in 3 years.5,6 These findings align with a meta-analysis by Corrao et al. that showed that RA was significantly associated with aortic valve thickening and/or calcification and aortic valve stenosis.7 The study also found that patients with established RA had a five times greater risk of developing aortic valve stenosis compared with controls. Regular monitoring of patients with established RA, even at a young age, is recommended to track the progression of aortic valve insufficiency to stenosis.
The possible cause of aortic stenosis can also be determined by histopathology. Peyrou et al.’s case report showed that the presence of rheumatoid nodules in the aortic valve of a 67-year-old woman with RA and aortic stenosis was consistent with research that notes the most distinct valve lesions in RA are rheumatoid granuloma within valve leaflets, resulting from non-specific inflammatory and fibrotic reactions at the base of the valve, leading to thickening and calcification.6 However, these findings are not conclusive evidence of RA.
A patient with established long-standing RA who had calcific aortic stenosis was found to have fibro-collagenous to fibro-hyalinised tissue with myxoid degeneration, dystrophic calcification and osseous metaplasia in the histopathology of the aortic valve, which confirms calcific aortic stenosis but is non-specific for RA.8
A recent systematic review found that RA raises the risk of cardiovascular death and reduces life expectancy. Furthermore, one study demonstrated that RA is a significant risk factor for patients who undergo SAVR.9 In a multicentre retrospective cohort study involving 109 patients, it was observed that patients with RA had higher all-cause mortality and a greater incidence of major cardiovascular events in long-term follow-up after SAVR. Short-term follow-up also revealed risks of cardiovascular events.10 Despite the increased risk of cardiovascular events brought by RA, there is still no consensus for treatment for patients with aortic stenosis and connective tissue disease such as RA. However, a 2021 study showed that TAVR is a viable solution even for patients with both connective tissue disease and high surgical risk.11 TAVR can provide significant decline in symptom burden while being less invasive compared with SAVR
Neurological complications are possible in patients who have had AVR and the patient in this case report experienced an acute infarct of the right frontal lobe within 2 hours of the procedure. Trials such as PARTNER 1A show that the risk of neurological events after SAVR is lower than TAVR at 2.4% and 4.3% at 30 days and 1 year, respectively.10 However, these studies did not specifically include patients with RA, who are at higher risk of calcification and progression of aortic stenosis and therefore may have a higher risk of postoperative neurological events.
The causes of stroke post AVR include violent manipulation of a calcified aorta and stenotic aortic valve, resulting in debris embolisation into the brain circulation. Factors that contribute to neurological complications in the early phase include female sex, chronic kidney disease, a history of stroke, peripheral vascular disease, low BMI, a history of falls, new onset AF, angina and an absence of a coronary artery bypass graft. Late-phase predictors include having a small body surface area, a degree of AV calcification, history of stroke, peripheral vascular disease and chronic AF. Our patient did not meet any of these predictors, but the uncontrolled RA leading to early onset severe calcific aortic stenosis could have contributed to the postoperative stroke.
A recent systematic review found that the risk of stroke in RA patients is significantly increased regardless of sex.5 Therefore, the inherent risk of stroke in RA patients combined with the risk of AVR would further increase the patient’s overall risk for stroke after the procedure.
Arrhythmias are a potential complication after AVR, but the incidence is unclear due to limited studies consisting of small case series and post hoc analyses of clinical trials. A variety of arrhythmias, including AF, ventricular tachycardia and supraventricular tachycardia, can occur post AVR. New onset AF was noted in 50.1% of AVR hospitalisations and it was associated with increased mortality. Ventricular tachycardia was also noted in some patients and it could present either very early postoperatively or several years later. However, there are only a few case reports of supraventricular tachycardia post AVR.
The incidence of arrhythmias may be higher in patients with uncontrolled RA, so reducing inflammation may be the best way to prevent them. Our patient experienced ventricular tachycardia and supraventricular tachycardia on the first postoperative day, which could have contributed to an increased mortality risk, along with other factors such as infection and acute stroke.10
Conclusion
Severe calcific aortic stenosis, whether symptomatic or not, usually requires AVR to alleviate symptoms. While this typically affects older people, the patient in this case report presented a unique case of accelerated calcification caused by uncontrolled RA. This condition can hasten atherosclerosis and lead to aortic valve calcification at an earlier age.
Despite undergoing SAVR, this patient suffered from immediate postoperative complications such as stroke and arrhythmia. Calcification debris can embolise into the cerebral circulation and further increase the risk of stroke in patients with uncontrolled RA undergoing AVR. This patient also experienced repeated ventricular and supraventricular tachycardia, which can increase mortality rates and be caused by post-surgery inflammation and uncontrolled RA.
These factors highlight the importance of proper follow-up for systemic rheumatic conditions such as RA to mitigate their impact on the cardiovascular system. 
Clinical Perspective
- This case shows how a poorly managed rheumatological disease, such as rheumatoid arthritis, can lead to early onset high atherosclerotic and inflammatory burden such as severe calcific aortic stenosis.
- Management of the overall inflammatory state of the body is necessary to control cardiovascular events, especially in high-stress states, such as a cardiac valve surgery.
- Immediate postoperative stroke and ventricular tachyarrhythmias are some uncommon complications of poorly controlled rheumatoid arthritis and severe calcific aortic stenosis due to high atherosclerotic and inflammatory burden.