Several large studies have consistently demonstrated that lipid-lowering therapy with statins reduces cardiovascular risk, regardless of underlying coronary artery disease (CAD).1 Particularly in patients with stable CAD and acute coronary syndrome (ACS), statin therapy has been shown to achieve marked reductions in mortality and recurrent cardiac events.2–5 These data established very early clinical benefits that persisted on long-term follow-up. Furthermore, the PROVEIT-TIMI 22 and MIRACL trials have shown even better clinical outcomes with early, intensive statin therapy in ACS.3,5 Adherence to statin therapy in post-ACS patients is also well established as being directly related to statin initiation during the index admission.6
In Japan, the therapeutic target for LDL in patients after ACS was <2.59 mmol/l, but in 2017 the Japan Atherosclerosis Society (JAS) Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases changed the target to <1.81 mmol/l.7 Intensive lipid-lowering therapy, including the combination of a strong statin (atorvastatin, pitavastatin, and rosuvastatin), ezetimibe and a proprotein convertase subtilisin/kexin type 9 inhibitor (PCSK9i), is now recommended for high-risk post-ACS patients.7,8
In addition to advances in lipid-lowering therapy, interventional cardiology in ACS is also progressing, with the introduction of intravascular imaging and newer drug-eluting stents. We thought it was clinically important to determine the effects of more intensive lipid-lowering therapy in accordance with current guidelines in the clinical revascularisation setting.
Aim
The primary purpose of the present study was to evaluate the effects of the introduction of new Japanese dyslipidaemia guidelines on the prescription status (not only the intensity of statin treatment but also changes in statin and other lipid-lowering prescriptions) and lipid profile of patients with ACS in the real-world clinical setting. As a secondary aim, we also investigated the effect of more intensive lipid-lowering therapy on clinical outcomes.
Methods
Study Design and Patient Enrolment
The present retrospective observational cohort study was conducted at Kawasaki Medical School Hospital (Kurashiki, Japan). The KIBIDAN-Go (KawasakI BioImaging DAtabase for loNg term cardiovascular prognosis) Registry was reviewed and consecutive patients with de novo ACS admitted to Kawasaki Medical School Hospital between January 2012 and September 2019 were identified.
Using the June 2017 update of the Japan Atherosclerosis Society guidelines, patients were divided into two groups: those treated before (Group 1) and those treated after (Group 2) the guideline update. The exclusion criteria were a history of percutaneous coronary intervention (PCI) or coronary artery bypass surgery (CABG), loss to follow-up blood sampling and loss to follow-up 9–12 months after coronary angiography.
This study was approved by the Ethics Committee of Kawasaki Medial School Hospital (Approval no. 3919) and was conducted in line with the requirements of the Declaration of Helsinki. Informed consent was not required because this was a retrospective study in which all patient data were de-identified prior to use.
Baseline Characteristics and Angiographic Assessment
Comprehensive clinical data were collected, including background characteristics, blood samples, medications and angiography procedural information, from reviews of charts and operation records. Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg or the current use of antihypertensive medication. Dyslipidaemia was defined as serum LDL ≥3.62 mmol/l, HDL <1.03 mmol/l, triglyceride (TG) ≥1.69 mmol/ or the current use of cholesterol-lowering medication. Diabetes was defined as fasting plasma glucose ≥126 mg/dl, HbA1c ≥6.5% or the current use of antidiabetes medication. Smoking status was classified as either current smoker or ex-smoker.
The rates of intracoronary imaging-guided PCI and the use of second- or third-generation drug-eluting stents in the treatment of ACS were also evaluated. All patients underwent successful PCI on admission for the index ACS. Patients underwent follow-up examinations, blood sampling and coronary angiography after 9–12 months.
Qualitative and quantitative coronary angiography (QCA) was evaluated in accordance with the clinical expert consensus document from the Japanese Association of Cardiovascular Intervention and Therapeutics using CAAS 7 (Pie Medical Imaging).9 Baseline, post-procedure and follow-up angiograms were assessed in all patients for whom angiograms were available for analysis. The target segment was defined as the entire segment involving the implanted stent and the edges 5 mm proximal and distal to the stent. A segment treated with multiple overlapping stents was regarded as a single target segment.
The following parameters were obtained through QCA: lesion length, minimum lumen diameter, reference diameter, diameter stenosis (DS) and late loss. In this cohort, angiographic restenosis was performed if angiography during follow-up showed DS ≥50%. Angiographic progression in a non-culprit lesion was defined as a progression in DS ≥50%.
Lipid Profile and Prescription
High-intensity statins were defined as atorvastatin 20 mg/day, rosuvastatin 10 mg/day and pitavastatin 4 mg/day. Low-intensity statins were defined as atorvastatin 5–10 mg/day, rosuvastatin 2.5–7.5 mg/day, pitavastatin 1–2 mg/day, pravastatin 2.5–15 mg/day or fluvastatin 20 mg/day. The primary endpoint was the change in prescription status and lipid profile.
Clinical Follow-up and Outcomes
The first clinical outcome parameter was a composite of cardiac death and non-fatal MI; the second clinical outcome parameter was the incidence of coronary events, including events from culprit and non-culprit lesions. This study excluded staged PCI procedures, which were defined as planned interventions after the first catheterisation. Information was gathered on clinical outcomes from medical charts or phone interviews. All patients were followed retrospectively up to October 2020 to assess survival status and clinical events.
Statistical Analysis
Values are presented as mean ± SD for quantitative variables and as numbers and percentages for qualitative variables. Continuous data were compared between subgroups using Student’s t-test for normally distributed data and the Wilcoxon rank-sum test for data that were not normally distributed. Qualitative data were compared between subgroups using Pearson’s χ-squared test.
Propensity score matching (PSM) was used to control for differences between Groups 1 and 2. Specifically, 1:1 nearest-neighbour matching was performed based on estimated propensity scores, with a calliper of 0.25 imposed to ensure that matching was within the zone of common support. After PSM, Pearson’s χ-squared test and, where appropriate, Fisher’s exact test were used to compare rates of the patient and angiographic lesion variables described above to ensure these factors were statistically equivalent between the two groups. After PSM, a Cox proportional hazard model was used to compare events.
All statistical analyses were performed using JMP version 15 (SAS Institute). Two-tailed p<0.05 was considered statistically significant.
Results
Clinical Characteristics
Among the total patient population, 274 patients were excluded because they did not meet the inclusion criteria: 109 patients had history of previous PCI, 18 patients had history of CABG, 122 patients were lost to follow-up CAG and 20 patients were lost to follow-up blood tests. Thus, 283 consecutive patients with de novo ACS were enrolled in the study (Supplementary Material Figure 1). Patients were allocated to either Group 1 (treatment before the guideline update; n=182) or Group 2 (treatment after the guideline update; n=101) depending on the onset of the index ACS. During the observation period (up to October 2020), the overall median clinical follow-up period was 788 days (interquartile range [IQR] 385–1,293 days).
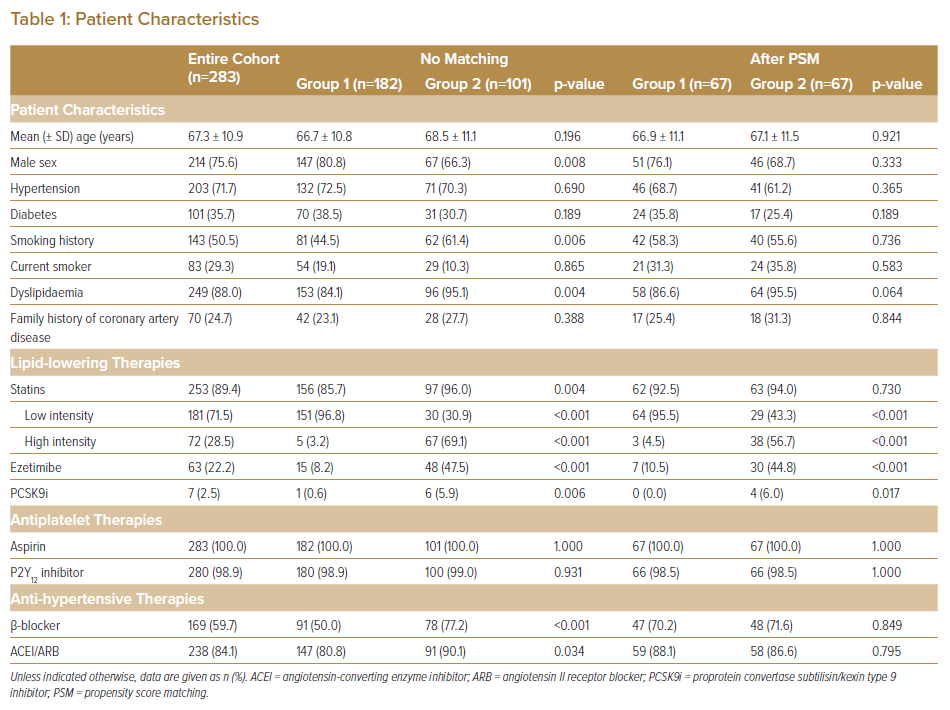
At baseline, mean age was 67 ± 11 years. Of the subjects, 214 (76%) were men. Significant differences were seen between Groups 1 and 2 in sex and dyslipidaemia. The percentage of patients using β-blockers and angiotensin-converting enzyme inhibitor (ACEI)/angiotensin II receptor blockers (ARBs) was significantly higher in Group 2 than in Group 1 (Table 1).
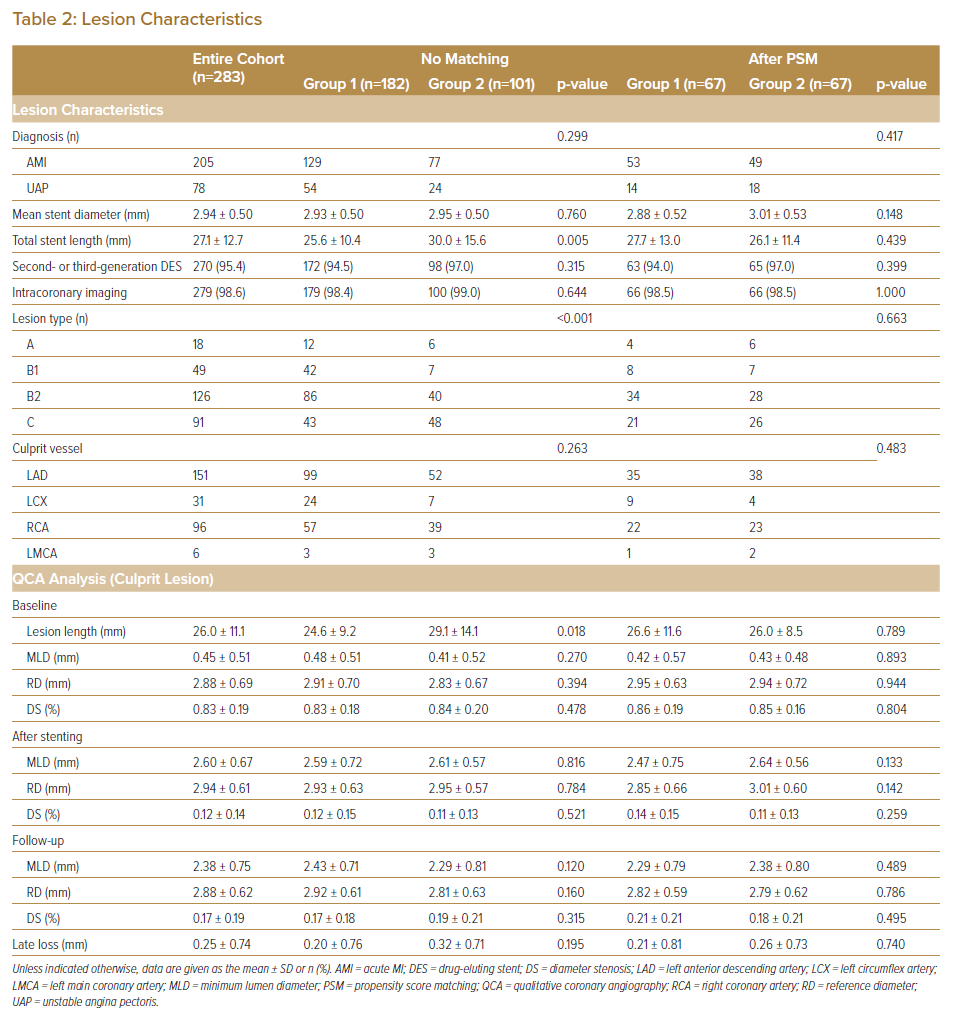
Lesion characteristics (lesion length, lesion type and total stent length) differed significantly between the two groups (Table 2). The rates of imaging-guided PCI and the use of second- and third-generation drug-eluting stents in the treatment of ACS were >90% in both Groups 1 and 2, with no significant differences between (94.5% versus 97.0% [p=0.315] and 98.6% versus 99.0% [p=0.644], respectively). After PSM, all categorical variables (age, sex, smoking status, the use of β-blockers and ACEI/ARB, total stent length, lesion type and follow-up period) were acceptably matched between the two groups.
Lipid Profiles and Prescriptions
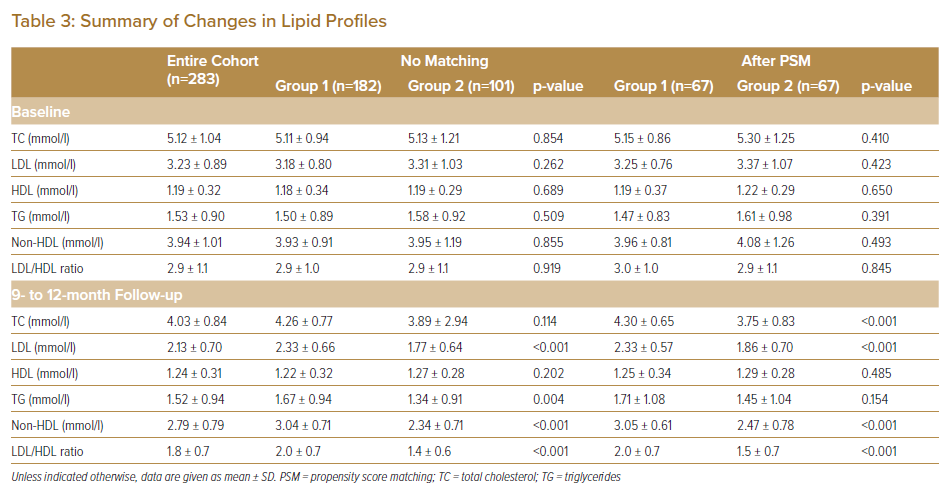
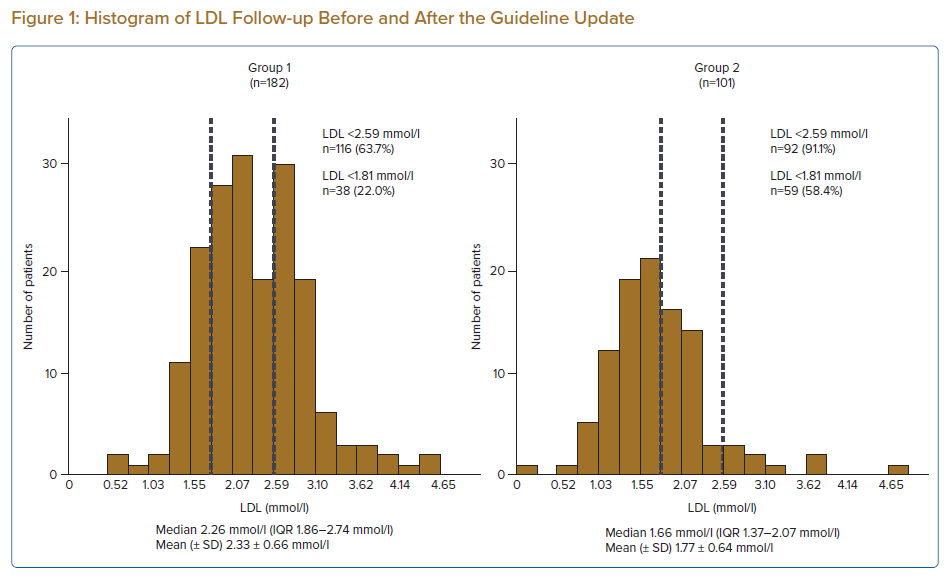
The percentage of patients receiving lipid-lowering therapies was significantly higher in Group 2 than in Group 1. The statin adherence rate was 98.4%. All cases of discontinuation occurred in Group 2. The reasons for statin discontinuation included liver dysfunction (n=1), digestive symptoms (n=1) and poor adherence (n=2). No significant differences in baseline lipid profiles were apparent between the two groups. Conversely, LDL and TG at follow-up were significantly lower and non-HDL was significantly higher in Group 2 (Table 3). The distribution of follow-up LDL concentrations before and after the guideline update is shown in Figure 1. Before the guideline update, only 22.0% of patients achieved an LDL target value of <1.81 mmol/l, with 63.7% of patients achieving an LDL target value of <2.59 mmol/l. However, after the guideline update, 58.4% of patients achieved an LDL target value of <1.81 mmol/l.
In Group 1, only one-quarter of patients treated with low-intensity statins alone and half the patients treated with low- or high-intensity statin plus ezetimibe achieved the new LDL target. In Group 2, 85% of patients who were treated with the combination of high-intensity statin plus ezetimibe therapy achieved the LDL target. The proportion of patients achieving the target LDL using the combination treatment tended to be higher in Group 2 than in Group 1 (84.9% versus 50.0%, respectively; p=0.268; Supplementary Material Figure 2).
Clinical Outcomes
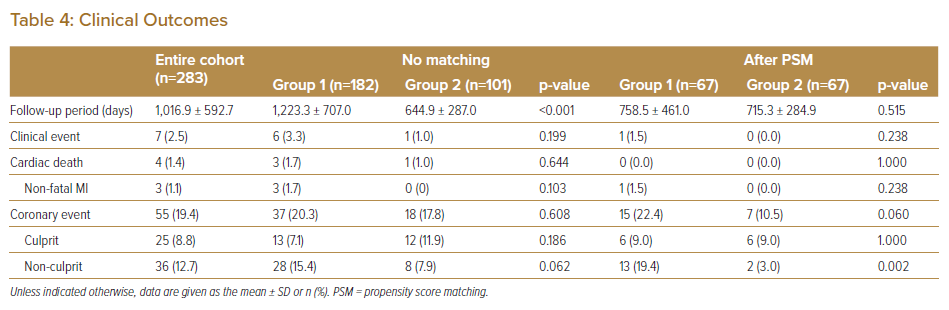
During follow-up, seven patients (2.5%) developed clinical events and 55 patients (19.4%) developed coronary events. The median follow-up was 1,100 days (IQR 546–1,603 days) for Group 1 and 580 days (IQR 365–902 days) for Group 2. Before PSM, there were no significant differences in clinical and coronary events between the two groups (Table 4).
After PSM, the incidence of coronary events from non-culprit lesions was significantly lower in Group 2 than in Group 1 (3.0% versus 19.4%, respectively; p<0.01). There were no significant differences in the rates of clinical and culprit lesion events between the two groups. Using the Cox model revealed that the guideline update resulted in a lower rate of events from non-culprit lesions (HR 0.50; 95% CI [0.33–0.75]).
Discussion
The major findings of this study are that: after the introduction of the new guidelines, although post-treatment LDL levels were significantly improved, target LDL levels of <1.81 mmol/l were achieved by 58.4% of patients; after the introduction of the new guidelines, 85% of patients who were treated with combination high-intensity statin/ezetimibe therapy achieved target LDL levels; and the change in the guideline for more intensive lipid-lowering therapy resulted in a decreased rate of non-culprit lesion progression in patients after ACS.
Intensive lipid-lowering therapy, consisting of the combination of a strong statin, ezetimibe and PCSK9i, is now recommended for secondary prevention in high-risk patients after ACS. To the best of our knowledge, the present study is the first single-centre retrospective analysis to investigate the effect of guideline-compliant intensive lipid-lowering therapy on the lipid profile and secondary coronary events in patients after ACS.
As described previously, there is a close relationship between the LDL concentration achieved and coronary plaque regression, with the cut-off point at which coronary atherosclerotic development changes from plaque progression to regression being an LDL concentration of approximately 1.94 mmol/l.10 In patients with plaque regression, LDL concentrations achieved are significantly lower than in patients with plaque progression.10 Every 5 years, the JAS publishes guidelines for the treatment of dyslipidaemia and atherosclerosis. In accordance with the latest JAS (2017) guideline for secondary prevention in patients with familial hypercholesterolaemia, ACS and diabetes complicated by other high-risk conditions, such as non-cardiogenic cerebral infarction, peripheral artery disease, chronic kidney disease, metabolic syndrome, an overlap of major risk factors or smoking, stricter LDL control should be considered, with a concentration of <1.81 mmol/l set as the target.7
The REAL-CAD study, the first large-scale clinical trial in Japan, showed that high-dose pitavastatin (4 mg/day) significantly reduced major adverse cardiovascular events (MACE) compared with low-dose pitavastatin (1 mg/day).11 Based on the results of the REAL-CAD study and a previous intravascular ultrasound trial, which showed coronary plaque regression using high-dose statins, the maximum tolerable dose of a high-intensity statin is recommended as first-line therapy for ACS patients, regardless of pre-intervention LDL concentrations.10 A systematic review demonstrated that combination therapy comprising of statin/ezetimibe and statin/PCSK9i (particularly with high-intensity statins) could help attain the previously recommended strict LDL goals of <1.81 mmol/l.12 The results of the present study confirm that LDL target goals could be achieved following combination therapy with a high-intensity statin and ezetimibe. In addition, with LDL <1.81 mmol/l as the target, reductions in cardiac events were demonstrated in the IMPROVE-IT, FOURIER, ODYSSEY OUTCOMES trials.13–15 Using the same LDL target, the GLAGOV and PRECISE- IVUS trials showed plaque regression.16,17
In the present study, we confirmed that intensive lipid-lowering therapy decreased the rate of non-culprit lesion progression in patients after ACS. Conversely, we did not find any significant differences in the rate of in-stent restenosis between the two groups. This may represent the effect of advances in drug-eluting stents.
Current guidelines from the Japanese Circulation Society (JCS; issued in 2018), the American College of Cardiology/American Heart Association (ACC/AHA; issued in 2018) and European Atherosclerosis Society/European Society of Cardiology (EAS/ESC; issued in 2019) regarding the treatment of hyperlipidaemia place particular emphasis on patients with very high cardiovascular risk and recommend stringent LDL lowering with statins.8,18,19 The JCS and ACC/AHA guidelines recommend fixed high-dose statins for high-risk patients with atherosclerotic cardiovascular disease (ASCVD), with the addition of ezetimibe to the maximum tolerated statin dose reasonable when the LDL concentration remains ≥1.81 mmol/l.8,18 Furthermore, EAS/ESC guidelines recommend a treatment goal of LDL <1.42 mmol/l or a >50% reduction in LDL.19 For patients with ASCVD who experience a second vascular event within 2 years (not necessarily of the same type as the first event) while taking the maximum tolerated dose of statin-based therapy, an LDL goal <1.03 mmol/l may be warranted.19
However, there is insufficient evidence to recommend a target value of LDL <1.42 mmol/l among Japanese ACS patients.
Recently, a subanalysis of the FOURIER trial suggested that patients who achieved the lowest LDL values had the lowest risk of future MACE.20 The clinical outcomes in the present study were comparable to those in that trial, but we recognise that our findings regarding clinical outcomes are not conclusive, but rather hypothesis generating, because of several study limitations (see below). However, we think it is important to elucidate the effects on ACS patients of intensive lipid therapy based on the newly revised guideline and in the current clinical revascularisation setting.
Limitations
Some potential limitations to our data need to be considered. First, although we adjusted for known confounders, the data are observational in nature and inherently subject to residual confounding. For example, detailed haemodynamic, echocardiographic and intracoronary imaging data could not be extracted from the dataset.
Second, patients were retrospectively and not randomly selected from a single centre, and the relatively small number of patients with selection biases may have affected the results. Although PSM can be used to adjust for measured independent variables, it sometimes struggles when there is a limited number of patients. Therefore, the results in the present study are for an exploratory analysis, with further investigations using data with greater accuracy required.
Third, the results of this study are applicable only to Japanese patients treated under the Japanese healthcare system. It may not be possible to generalise the results to other populations under different healthcare systems in other countries. For example, atorvastatin 20 mg/day, rosuvastatin 10 mg/day and pitavastatin 4 mg/day are classified as mild-intensity therapies in the US and Europe.18,19 However, the approved starting dose for aggressive lipid-lowering therapy in the present study was for Japanese patients, who generally have a lower body weight than non-Asian populations.
Finally, the follow-up period differed between the two groups because the guidelines were revised in 2017.
Conclusion
The introduction of new Japanese dyslipidaemia guidelines significantly improved the prescription status and lipid profile of patients with ACS, but almost 50% of ACS patients still did not achieve target LDL concentrations. Guideline-oriented, more intensive lipid-lowering therapy may decrease events from non-culprit lesions in patients after ACS.
Click here to view Supplementary Material.
Clinical Perspective
- This study evaluated the effect of changes in Japanese guidelines for dedicated, intensive lipid-lowering therapy on lipid profile and secondary clinical outcomes in patients with acute coronary syndrome (ACS).
- Most patients who received combination intensive statin/ezetimibe therapy achieved a target LDL of <1.81 mmol/l.
- The guideline change for dedicated, intensive lipid-lowering therapy resulted in a decrease in the rate of non-culprit lesion progression in patients after ACS.