Stroke remains a significant cause of morbidity and mortality after cardiac surgery. AF is the most common clinically important cardiac arrhythmia, occurring in approximately 0.4–1% of the general population and increasing with age to >8% in those >80 years of age. One underlying reason for stroke is postoperative AF (pAF), occurring in approximately 0.4–1% of the general population.1 pAF is considered an independent predictor of various adverse outcomes, including a two- to fourfold increased risk of stroke and a twofold increase in all-cause mortality. In the US, it is independently associated with excess length of hospital stay and hospitalisation costs related to treatments and days in hospital.2
The presence of pAF predisposes the formation of atrial thrombus. Based on observation, 90% of the thrombi found in nonvalvular AF patients and 57% found in valvular AF are located in the left atrial appendage (LAA), which is a significant source of cardioembolic stroke.3 Approximately 14–30% of all cerebral infarctions are cardioembolic stroke.4 Formation often occurs within the LAA because of reduced contractility and stasis, leading to AF thrombus resulting in cardioembolic stroke.
Current primary care guidelines recommend the administration of direct oral anticoagulants (DOACs) as the preferred therapy to prevent stroke in patients with AF and a CHA2DS2-VASc ≥2.5,6 The 2016 European Society of Cardiology guidelines state that oral anticoagulation (OAC) for patients with nonvalvular AF is associated with an increased risk of stroke (class 1 indication; level of evidence a) and the preference to use DOACs over vitamin K antagonists in these patients.7 According to Miller et al., DOACs showed a decreased risk for all-cause stroke and systemic embolism over warfarin in patients with AF.8
Despite the benefits of OACs, they cannot be offered in all patients who are poor candidates for long-term OACs because of the high risk of bleeding, drug adherence and drug tolerance. Lambert et al. reported that of all included patients, 4.5% (2,418) experienced a major bleeding event during total follow−up (HR 1.23; 95% CI [1.05–1.43]). Specifically, the 30-day significant risk of bleeding was 0.8%, with the most common bleeding sites being gastrointestinal, renal and intracranial (HR 1.41; 95% CI [1.01–196]).9
The observation that most thrombus formations are located in the LAA triggered significant interest in the LAA as a potential therapeutic target. This is based on several studies that suggest that concomitant LAA occlusion (LAAO) may be performed as an adjunctive procedure during cardiac surgery.10–21 According to the 2019 American Heart Association/American College of Cardiology and Heart Rhythm Society, surgical occlusion of the LAA may be considered in patients with AF undergoing surgery (class 2b indication; level of evidence B-NR).22
In a recent meta-analysis by Ibrahim et al., 13,352 adult patients with AF were studied, revealing that surgical occlusion of the LAA group was associated with a reduced risk of stroke, supporting the assumption of concomitant LAA in preventing stroke for patients with AF.23
Despite recent trials, the evidence for routine use of LAAO during elective open-heart surgery remains controversial. We performed this meta-analysis to assess the efficacy and safety of LAAO to prevent ischaemic stroke among adult patients with a history of AF during concomitant cardiac surgery, documenting all-cause mortality and the risk of AF recurrence.
Methods
Literature Search
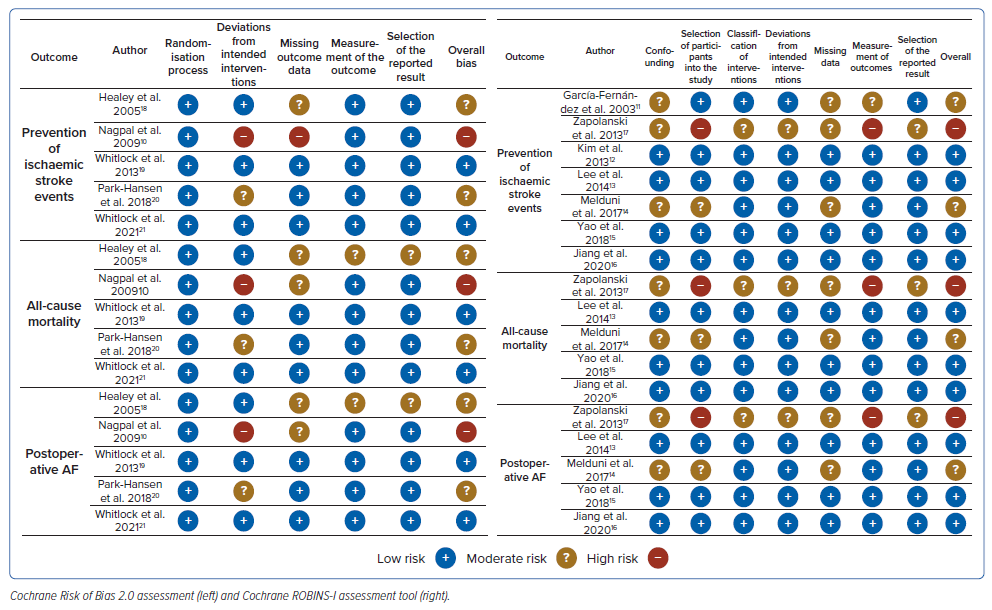
This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. We performed an electronic search of the National Library of Medicine, Cochrane, The Lancet, Clinical Trials.gov, and Science Direct to and including October 2021 using the keywords “left atrial appendage occlusion”, “systemic embolism” and “stroke.” The process of searching and reviewing was performed by two independent evaluators (RET, BJD) and discrepancies were discussed with a third expert investigator (CST). The search strategy is shown in Figure 1.
Definitions and Data Extraction
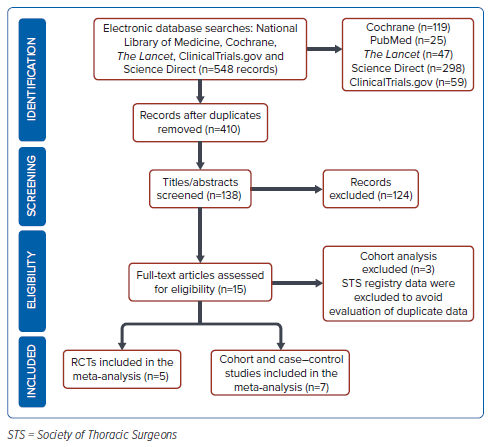
Studies that compared all forms of cardiac surgery performed with or without LAAO were selected. Studies were included if the following criteria were met: subjects underwent cardiac surgery with concomitant LAAO; transoesophageal echocardiogram was performed to document the exclusion of the appendage; stroke occurrence was assessed by imaging using cranial MRI or questionnaires validating a stroke status. Studies excluded from the meta-analyses were as follows: abstracts without full-text publication; the absence of the above-mentioned clinical outcomes; studies from the national inpatient sample and Society of Thoracic Surgeons registry to prevent duplication. Participants who underwent off-pump surgery, pacemaker or any metallic device implantation, heart transplantation, complex congenital heart surgery or had haematological problems or active endocarditis were also excluded. There was no restriction on the language of reporting or the period. Published and unpublished randomised clinical trials were included, whether they were single-blinded, double-blinded, or unblinded. The primary outcome measure in this study was the prevention of stroke after LAAO. Secondary outcome measures included all-cause mortality and pAF.
Data Collection
The review authors planned the relevant data to be collected in this meta-analysis and systematic review. A data collection form was created, including the citation details, study design, total study duration, type and number of participants, study location, study inclusion and exclusion criteria, baseline patient characteristics, description of the intervention and control, the relevant outcome of interest and results.
Statistical Analysis
Meta-analysis using both random and fixed-effects models was performed to determine the pooled effect estimates. The effect measure of choice for the outcome was reported as the risk ratio (RR) for dichotomous data and 95% CI. The Mantel–Haenszel method was used primarily in studies with small sample sizes and lower event rates. Assessment of bias was completed on all included studies using the Cochrane Risk of Bias (RoB) 2.0 tool for randomised controlled trials and the ROBINS-I tool for non-randomised studies. Sources of heterogeneity were assessed using a homogeneity test based on the X2 test. The I2 statistic was used to assess statistical heterogeneity across studies. An I2 value of 30–60% was considered moderate heterogeneity, 50–90% substantial heterogeneity and 75–100% considerable heterogeneity. A fixed-effect estimate was used, but a random-effects analysis model was used if heterogeneity was noted. Evidence of publication bias was evaluated using a funnel plot, and the relationship of the LAAO and stroke occurrence between studies was determined through L’Abbe plot. In the presence of significant heterogeneity despite the random-effects model, a sensitivity analysis was performed by exclusion of studies ineligible based on sample size, methodological quality, population variance and intervention features. The certainty of evidence was assessed using the Grades of Recommendation, Assessment, Development, and Evaluation Working Group (GRADE) approach.24 All statistical analyses were performed using RevMan 5.4. Two-tailed p<0.05 was considered statistically significant.
Results
Study Selection
Of the 548 records identified through database searching, 410 duplicate records were removed, and 124 articles were excluded after screening the titles and abstracts because of inappropriate population, publications, and study design. Of the remaining 15 studies, three articles were excluded as the population were gathered from the Society of Thoracic Surgeons Adult Cardiac Surgery national registry, finally yielding 12 studies with 18,982 patients for final analyses.
Study Characteristics
Baseline characteristics of the study populations are reported in Table 1. Among the 12 studies, there were five randomised controlled trials, six cohorts, and one case–control study. The mean age ranged from 53.4 to 65.2 years, with predominantly men being included. Post-operative follow-up ranged from 1 month to 9 years. All participants involved underwent cardiac surgery with LAAO, although the type of procedure varied widely.
Pooled Incidence of Ischaemic Stroke and All-Cause Mortality
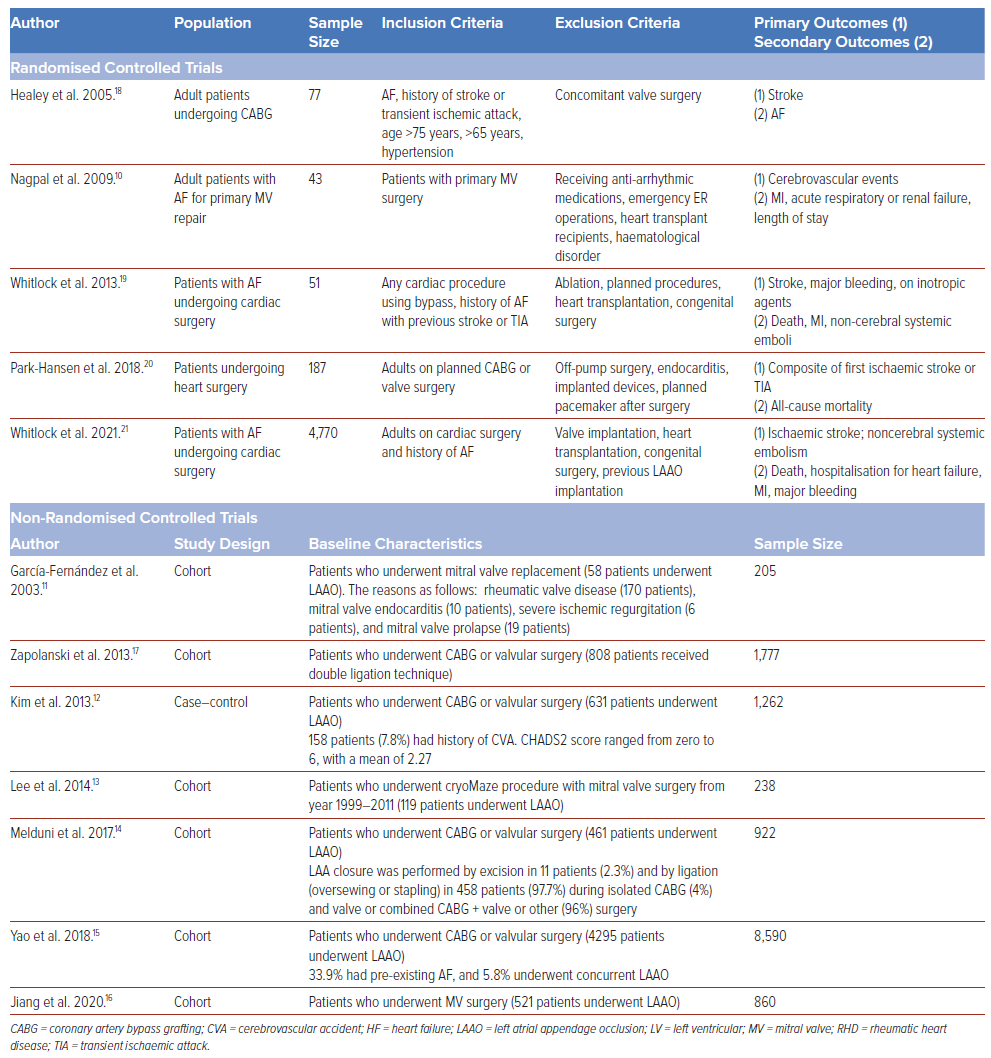
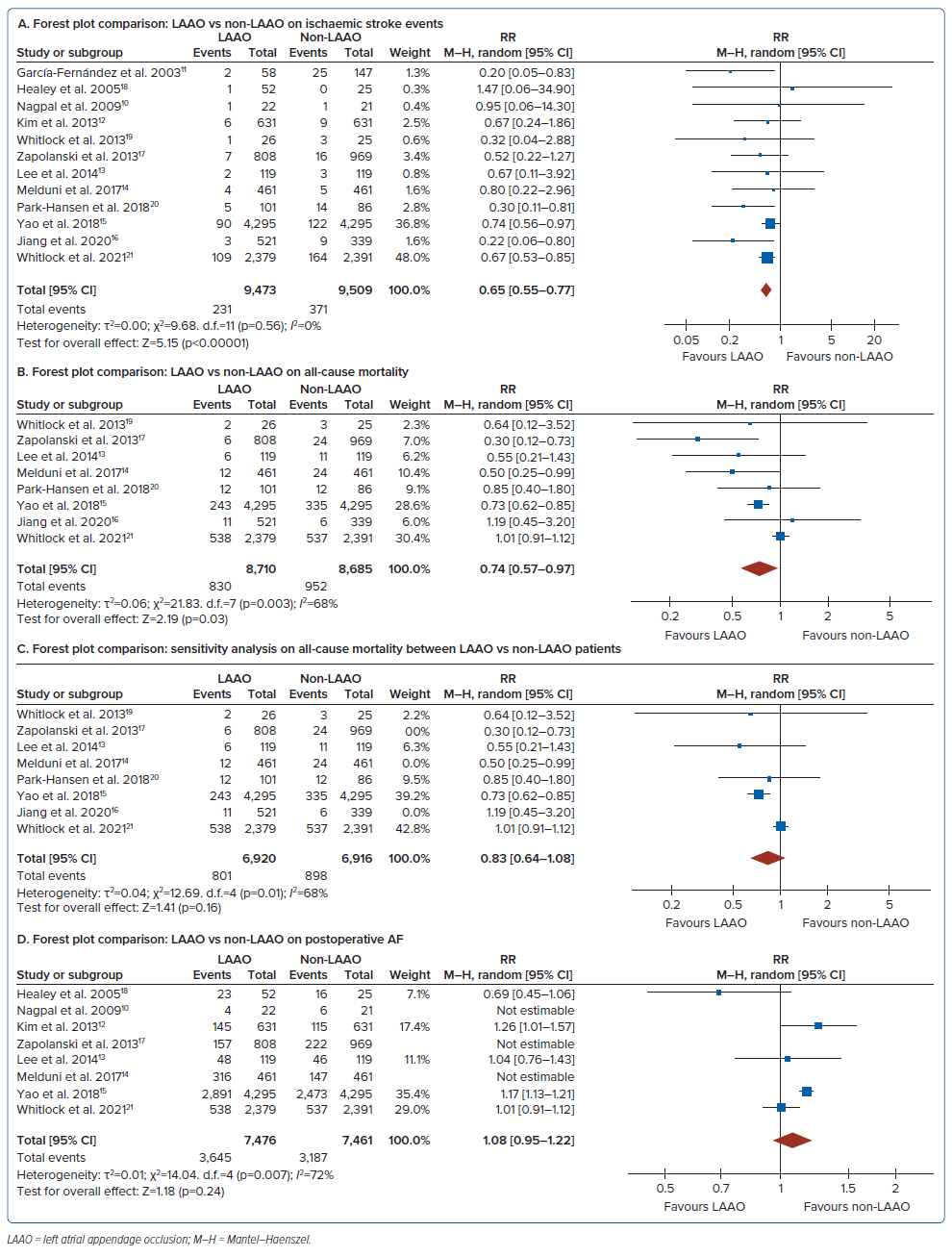
The RR of having an ischaemic stroke event in concomitant LAAO was 0.65; 95% CI [0.55–0.77], whereas all-cause mortality was 0.74; 95% CI [0.57–0.97] (Figure 3A and B). Between-trial heterogeneity was identified in this analysis, with an I2 of 68% as reflected by the L’Abbe plot (Figure 2).
Sensitivity Analysis on All-Cause Mortality and Postoperative AF
A study by Zapolanski et al. had a high risk of bias based on the ROBINS-I tool.17 The exclusion of this study showed the same value for heterogeneity (Figure 3C), still with moderate heterogeneity.
Another secondary outcome showed a 1.09-fold risk of pAF in the LAAO group but not statistically significant (RR 1.08 [95% CI [0.95–1.22] p=0.24, I2 = 72%; Figure 3D). The major sources of heterogeneity identified were variations in surgical techniques; three trials demonstrated several left atrial occlusions (amputation, closure via surgical or stapler device) and the long-term follow-up, which was inadequate among the included studies.19–21 There was only one study that documented outcomes completely after the first 30 days postoperatively.21
Quality Assessment
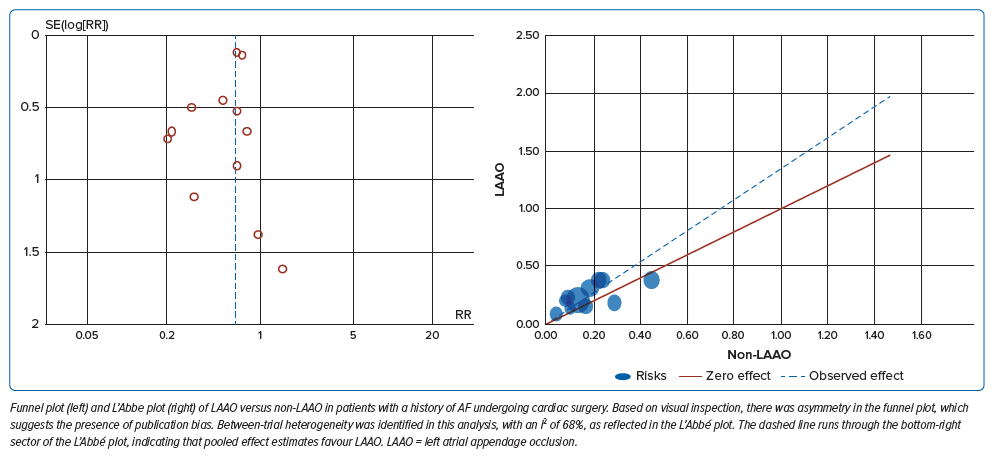
The full risk of bias assessment of individual trials is summarised in Figure 4. The RoB 2.0 and ROBINS-I tools consist of a set of domains with a series of questions to develop a judgement that can be from “low”, “high” or with “some concerns”. Two studies had a high risk of bias due to incorrect participant selection, deviations from the pre-specified plan, missing outcome data and variable measurement of outcomes.
Certainty of Evidence
A GRADE approach was used for the primary outcomes of the study (Supplementary Table 1). The outcome for ischaemic stroke and all-cause mortality showed moderate certainty of evidence, which was due to allocation concealment, the randomisation process and underestimation of the actual effect.
Reporting Bias
We assessed publication bias for concomitant LAAO of patients undergoing cardiac surgery, which revealed asymmetry by visual inspection. Egger regression and Begg and Mazumdar tests were further performed using a two-tailed p-value indicating no evidence of bias (p=0.362 and p=0.21, respectively).
Discussion
This systematic review evaluated evidence from 12 studies (five randomised controlled trials, six cohort studies and one case–control study) involving 18,982 patients with a history of AF undergoing cardiac surgery from 2003–2021.
The initial hypothesis of LAAO in preventing ischaemic stroke arose from the postulate that most thrombus formation of AF is mainly located in the LAA as observed from previous studies.25,26 This structure, a remnant of the primordial embryonic left atrium, is considered to be non-functional. However, based on the study by Khurram et al., the association of the risk of AF depends on the LAA morphology, particularly on the degree of trabeculations.27 Extensive trabeculation is considered to be an independent risk factor to develop thromboembolic events (27.7% versus 14.4%; p=0.01).
Several previous studies have demonstrated that LAAO reduces the risk of ischaemic events and thromboembolism. In this meta-analysis, the result showed a risk of 0.65 times more likely to have an ischaemic stroke event (RR 0.65; 95% CI [0.55–0.77]; p<0.00001; I2=0%; moderate certainty of evidence). Despite the inclusion of studies that have a small sample size, there was no evidence of publication bias (Egger regression p-value 0.362, Begg and Mazumdar p-value 0.337). Documentation of the embolic events was mostly done through clinical parameters and guided questionnaires. Only two studies, Park-Hansen et al. and Whitlock et al., used cranial imaging for stroke documentation.20,21 There have been different surgical LAA techniques that may impact study results. In a study by Nagpal et al., there was a persistent risk of cardioembolic phenomenon with incomplete exclusion or appendage remnant of >1 centimeter.9 In a study by Zapolanski et al., thrombi developed in 41% of partially closed LAA.17
Regarding all-cause mortality, those who underwent concomitant LAAO had 0.74 times the risk of mortality than those without LAAO. Hence, this study showed that there were lower events of overall deaths in patients underwent surgical LAAO. In a 2019 meta-analysis by Ibrahim et al., the surgical LAAO cohort also had lower events of all-cause mortality (pooled OR 0.74; 95% CI [0.55–0.99]; p=0.0408) compared with the non-LAAO cohort. Thus, it may also support the safety of performing LAAO.23 The results of both studies are similar. The relationship between LAA and thromboembolic events with history of AF has revolutionised different techniques in modifying LAA surgically.28 However, different techniques and variations of LAAO may contribute to the heterogeneity of the studies regarding all-cause mortality.
In patients who develop pAF after cardiac surgery, it usually occurs within 2–4 days after surgery, with peak incidence on day 2. Predisposing factors involved were increased age or previous history of AF.29 In this meta-analysis, it showed no difference between the LAAO versus non-LAAO groups. There are several issues accounting for high heterogeneity of this outcome. Some involved studies could not account for the baseline characteristics of patients producing conflicting results. For patients without a prior history of AF, performing LAAO may lead to further complications and subsequent all-cause mortality. In Melduni et al., pAF was more common in patients receiving LAAO and was persistently observed after discharge, attributed to an increased number of hospitalisations compared with non-LAAO patients.14 The inadequacy of long-term follow-up in some studies may cause underestimation of pAF events.
Strengths and Limitations
This study presents a comprehensive systematic review and meta-analyses of concomitant LAAO versus non-LAAO in adult patients (≥18 years old) with a history of AF undergoing cardiac surgery. A systematic critical appraisal of included studies was performed using the updated Cochrane RoB 2.0 tool for randomised controlled trials, the ROBINS-I tool for the non-randomised studies and the application of the GRADE framework to determine the certainty of evidence. The study provides a systematic and detailed analysis of the assessment of the quality of evidence. However, given that the included studies are a combination of randomised and non-randomised trials, there is substantial heterogeneity among them, with observed variability in clinical characteristics, methodological design and quality, thus significantly affecting the certainty of evidence. The lack of randomisation and allocation concealment, high susceptibility to selection bias and uncontrolled confounders from non-randomised studies, if not carefully assessed, may impact the bias and clinical heterogeneity.
Most randomised controlled trials have small sample sizes, resulting in an underestimation of the effect of specific clinical outcomes. The study by Whitlock et al. is the only randomised controlled trial with a large sample size compared with other tests.21 Hence, a favourable outcome of the experimental treatment might be expected, contributing to clinical heterogeneity. Another limitation of this study is the absence of specification of baseline characteristics for AF, which could affect the occurrence of pAF. Moreover, a lack of information regarding the anticoagulation status of participants may have affected the rate of stroke. Some studies relied on the physicians’ discretion in administering antithrombotic therapy, resulting in an overestimation of effect. Underuse of anticoagulants may have contributed to stroke or overuse, resulting in higher bleeding risks, leading to increased all-cause mortality. Variations in the surgical techniques performed among studies might contribute to postoperative complications. Further studies are recommended to discuss and analyse each surgical method. The potential sources of bias in each study affect the magnitude of effect estimate remain elucidated.
Implications for Practice and Research
The overall certainty of evidence and effect estimate must be considered in assisting physicians in the clinical decision-making process in patient treatment. The cost-effectiveness of the procedure must also be considered.
Future research may require studies with improved methodological designs, appropriate statistical analyses, complete missing outcome data and bias correction. A meta-analysis of individual participant data or relevant subgroups may be implemented because of inconsistencies or heterogeneities in the studies. Performing LAAO in patients with a history of AF offers an additional reduction in the risk of stroke in addition to antithrombotic therapy but is not a replacement for OACs. Anticoagulation may have an additional benefit on ischaemic events or stroke prevention, but further trials are needed.
Conclusion
This meta-analysis showed that concomitant LAAO was more effective than no LAAO in preventing ischaemic stroke events and all-cause mortality, with an overall moderate certainty of evidence. However, it did not reduce pAF. We recommend concomitant LAAO for patients with AF who undergo cardiac surgery.
Clinical Perspective
- Stroke is considered to be a significant cause of morbidity and mortality after cardiac procedure and is mainly associated with AF.
- Left atrial appendage occlusion (LAAO) is considered a safe and effective treatment for stroke prevention in patients with AF.
- However, despite numerous data, LAAO remains underutilised because the current evidence is insufficient and requires further investigation.
- Concomitant LAAO has a great potential for preventing stroke in patients with AF who are poor candidates for long-term oral anticoagulation because of a high risk of bleeding, drug compliance and drug tolerance.
- LAAO has been shown to reduce all-cause mortality and ischaemic stroke events, although there was no change in post-operative AF.