Acute respiratory distress syndrome (ARDS) due to severe acute respiratory syndrome coronavirus 2 infection and the resultant heart–lung interaction could result in characteristic haemodynamic effects on the cardiovascular system.1 ECG, an easily accessible non-invasive method could be instrumental in categorising illness severity and predicting patient outcomes.
Materials and Methods
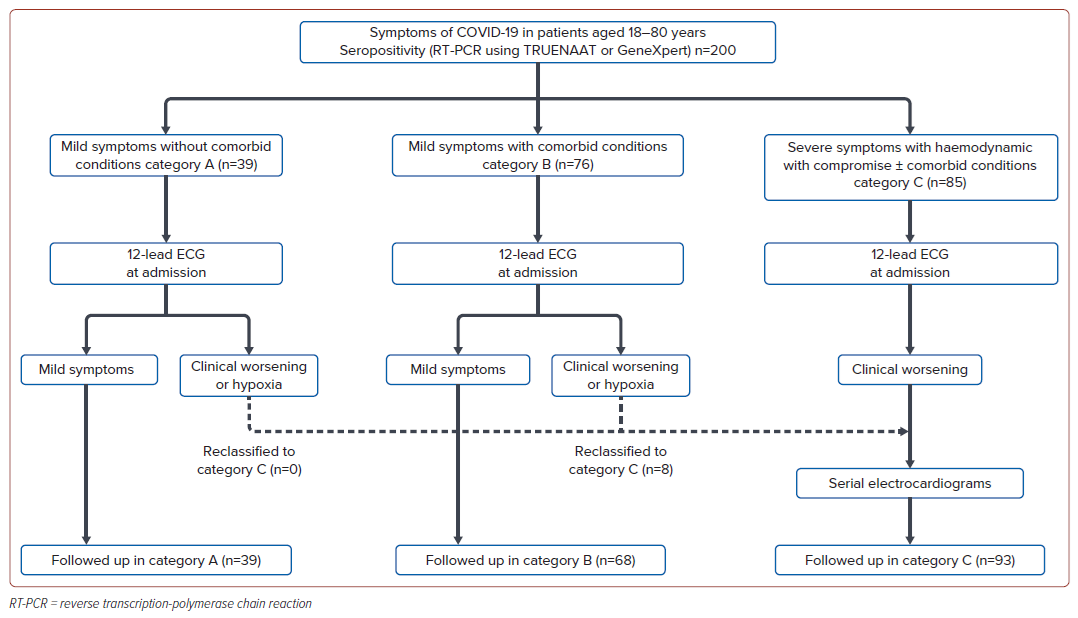
This prospective observational study was conducted at TD Medical College Hospital, Kerala, India, from June to November 2021, adhering to the Declaration of Helsinki with Ethics Committee approval (ECR/122/KL/RR-19-27/2021). It included 200 consecutive COVID-19 patients aged 18–80 years, diagnosed via real-time reverse transcription-polymerase chain reaction (real time RT–PCR) on the TRUENAAT or Gene-Xpert (Cepheid) platforms (Figure 1). We collected demographic and clinical data, grading disease severity into three categories based on symptomatology and risk factors: category A (mild symptoms), category B (mild symptoms plus risk factors, such as being aged >60 years, pregnancy, chronic conditions, malignancies or immunosuppressive therapy) and category C (severe symptoms with haemodynamic compromise).
The primary objective was to identify electrocardiographic findings significantly associated with COVID-19 severity. Secondary objectives included analysing predictors of adverse outcomes (mechanical ventilation or death) and examining serial ECG changes in critically ill patients. ECGs were taken upon admission to the intensive care unit (ICU) and during clinical deterioration or hypoxia, with daily follow-ups for critically ill patients. Categorisation was reassessed every 24–48 hours for patients in categories A and B who were upgraded to category C if they worsened clinically. ECG interpretation was performed by two independent cardiologists who were blind to patient details and study outcomes, using American Heart Association/American College of Cardiology/Heart Rhythm Society standardisation criteria.2 Patients were followed until discharge or death. Group differences were assessed using independent t-tests, ANOVA, Mann-Whitney, or Kruskal-Wallis tests based on data normality. Categorical data comparisons employed χ2 or Fisher’s exact test. Logistic regression identified independent outcome predictors with p<0.05 denoting statistical significance.
Findings
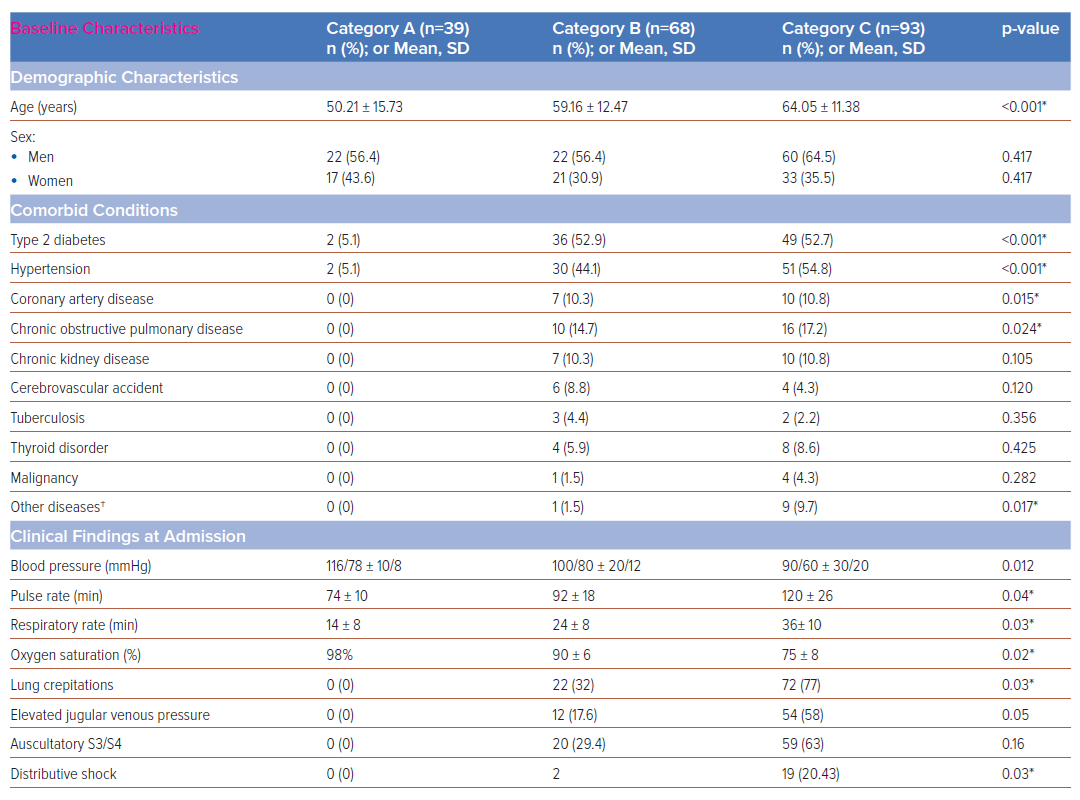
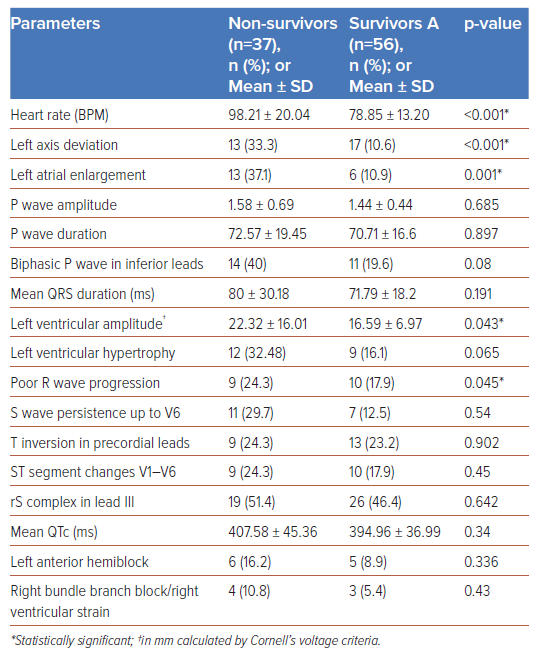
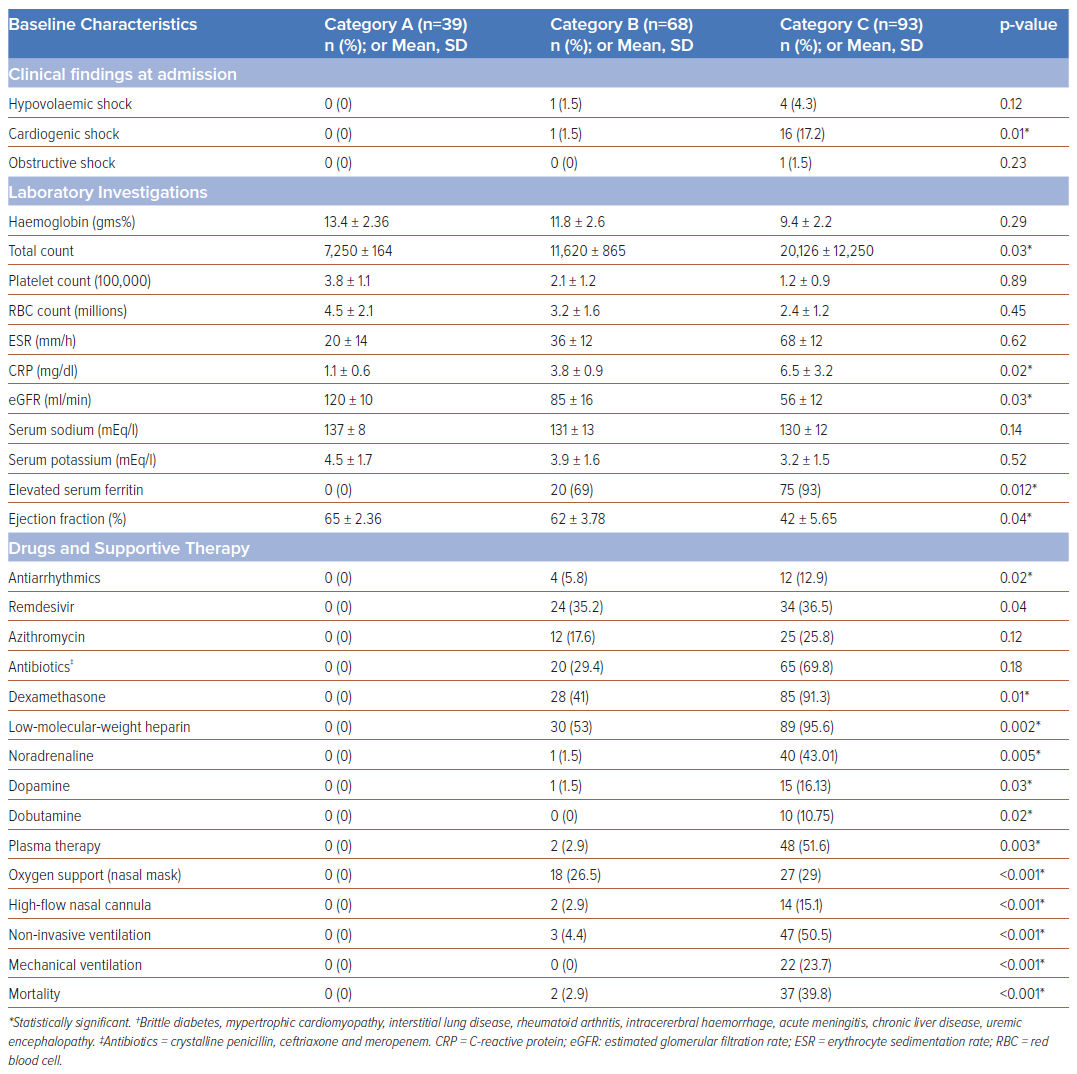
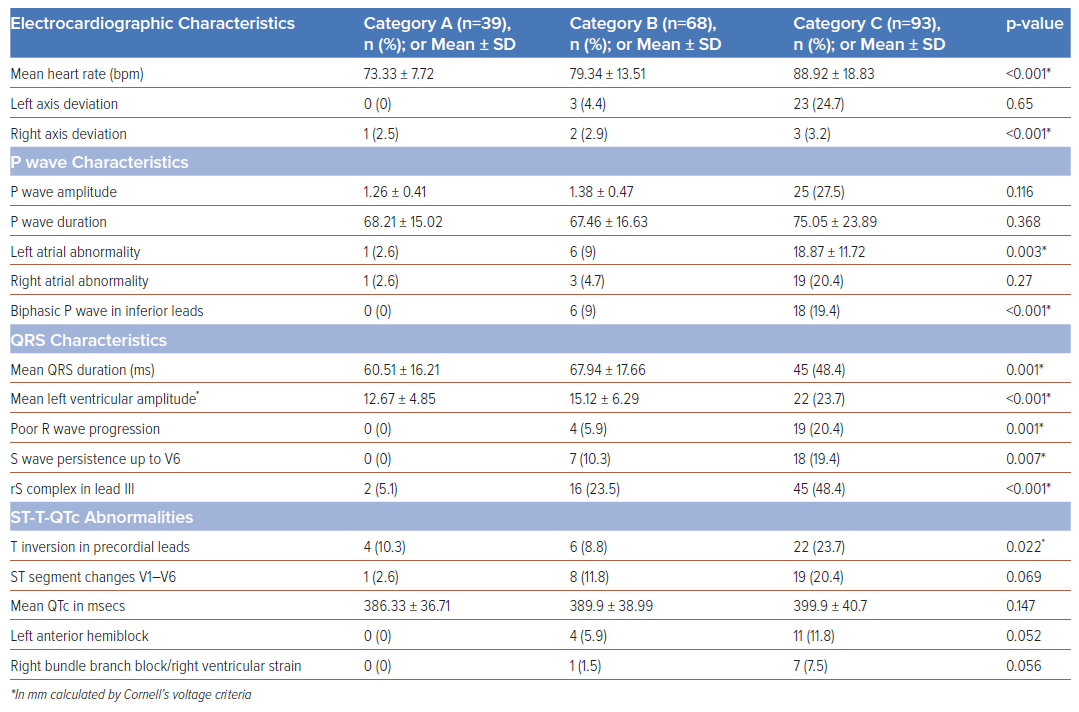
Of the 200 patients enrolled, 39 (19.5%) were category A, 68 (34%) were category B and 93 (46.5%) were category C. Baseline, clinical and electrocardiographic characteristics are summarised in Table 1. Category C patients exhibited significantly higher rates of heart failure, ARDS and elevated acute-phase reactants – erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) and ferritin – often requiring inotropic support and mechanical ventilation. Category C patients showed higher incidences of left axis deviation (p<0.001), left atrial enlargement (p=0.003), biphasic P waves in lead III (p<0.001), poor R wave progression (p=0.001), S wave persistence in V6 (p=0.007), and precordial lead T inversion (p=0.02) (Table 2). Of the 200 patients, 39 (19.5%) died, including 22 (23.7%) who were mechanically ventilated; and 15 (16%) in category C and two (2.9%) in category B who experienced sudden cardiac arrest. A novel electrocardiographic marker observed in 19 (48.7%) category C patients was a new-onset rS complex – R/S ratio >1 (p<0.001). In univariate analysis, heart rate (<0.001), left axis deviation (<0.001), left atrial enlargement (0.001), biphasic P waves in inferior leads (0.034) and left ventricular amplitude (0.043) were significantly associated with adverse outcomes (Table 3). Multivariate analysis showed age (OR 1.05; 95% CI [1–1.10]; p=0.046) and left atrial enlargement (OR 4.25; 95% CI [1.35–13.34]; p=0.013) as independent predictors of adverse outcomes.
Discussion
The electrocardiographic manifestations of COVID-19 depend on the pathophysiology of the disease – such as vascular endotheliitis, microangiopathy and intussusceptive angiogenesis – heart–lung interactions and haemodynamic effects on the cardiovascular system.3,4 Invasive catheterisation findings in mechanically ventilated COVID-19 patients show near-normal mean pulmonary artery pressure and pulmonary vascular resistance with standard right ventricular and atrial pressures.5 Notably, pulmonary hypertension typically presented a post-capillary pattern and an elevated left ventricular end-diastolic pressure (LVEDP) was observed, distinguishing severe COVID-19 cases from classical ARDS. The postulated mechanisms are blunted hypoxic pulmonary vasoconstriction, ventilation perfusion mismatch and intrapulmonary shunting.5 Consistent with the above observations, critically ill COVID-19 patients in our study had a higher incidence of left axis deviation, left atrial enlargement, primary ST-T changes in leads V1–V6 and poor R wave progression in precordial leads among critically ill COVID-19 patients with a very low incidence of right bundle branch block (RBBB)/right ventricular (RV) strain. These electrocardiographic abnormalities were more common in patients with advanced age, diabetes, systemic hypertension, coronary artery disease and chronic obstructive pulmonary disease (COPD), likely due to the exacerbation of pre-existing conditions or new abnormalities induced by COVID-19.
A notable electrocardiographic finding in our study was a new-onset rS complex (R/S ratio <1) in lead III in 48.6% of category C patients associated with primary repolarisation abnormalities in leads V3–V6 and clinical worsening (Figure 2A). The observed rS complex in lead III may signify an abrupt, inappropriate elevation in LVEDP, likely arising from primary viral myocarditis or worsened comorbidities. This affects the anterolateral region of the left ventricle, generating a net electrical vector directed away from lead III towards V4–V6 (Figure 2B) and could serve as a marker for imminent clinical worsening, characterised by ST segment T wave changes in V1–V6. Repolarisation abnormalities (QTc prolongation) are adverse effects of macrolide antibiotics, azoles, antimalarials and electrolyte imbalance, but QRS abnormalities with associated ST-T changes have not been reported. We believe the observed new onset rS complex in lead III was not due to drugs or dyselectrolytaemia. Biphasic P waves in inferior leads, a marker of atrial tachyarrhythmias, was observed in 38% of the patients who died.6 AF was the most prevalent arrhythmia observed in 27% of the study population.
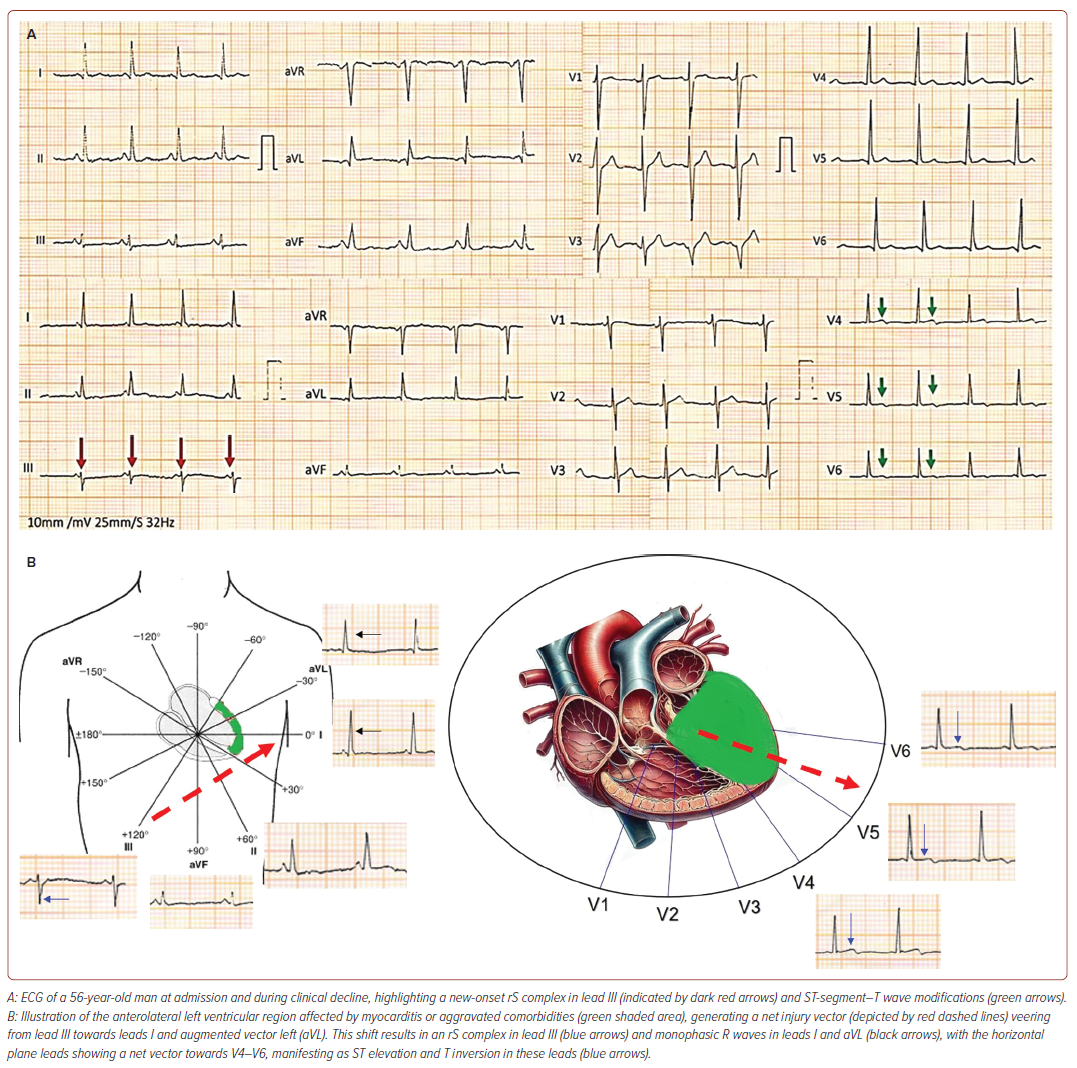
Serial electrocardiograms revealed that 73% of category C patients had a normal axis, with 24.7% exhibiting left axis deviation, which was significantly associated with higher mortality. Left axis deviation and left atrial enlargement correlated with a higher incidence of other abnormalities, such as S wave persistence in V6 and poor R wave progression, which affected patient outcomes. The authors hypothesise that the interplay between direct viral cytotoxic effects, worsening comorbidities and intrapulmonary shunting leads to a sudden inappropriate elevation in LVEDP, atrial stretch and increased pulmonary stiffness, eventually resulting in right ventricular failure. It is crucial to adjust observed pulmonary capillary wedge pressure (PCWP) for the external positive end-expiratory pressure (PEEP) in mechanically ventilated patients, as certain electrocardiographic abnormalities may stem from mechanical ventilation or non-invasive ventilation with high PEEP.
ECG abnormalities observed in COVID-19 patients encompass a wide range of issues indicative of myocardial stress, injury, or right ventricular strain. These include ST-segment elevation, T wave flattening, non-specific T wave inversions, QRS complex widening and poor R wave progression, often seen in severe respiratory cases such as multilobar pneumonia or significant pulmonary embolism.7 Conduction disorders such as high-degree atrioventricular blocks and transient complete heart blocks, possibly resulting from direct viral effects or antiviral therapy side-effects, are noted alongside left and right BBBs in up to 12% of patients.7,8 Cardiac arrhythmias, particularly sinus tachycardia, AF, atrial flutter, ventricular tachycardia and ventricular fibrillation, are prevalent in critically ill patients and serve as significant markers of adverse outcomes.8 Over 13% of patients exhibit prolonged QT intervals, which are linked to severe disease states requiring admission to intensive care and increased mortality, potentially exacerbated by medications such as chloroquine, hydroxychloroquine, and azithromycin.9 Additionally, morphologic changes, such as prominent R waves in early precordial leads and ST-segment depression with T wave inversion, suggest right ventricular strain. Finally, acute myocardial injury is evident, with about half of the patients showing ST-segment elevation at admission or during hospitalisation experiencing acute MI, while others present with non-occlusive myocardial injury.10
The majority of studies have observed that most ECG abnormalities in COVID-19 patients are repolarisation anomalies, with prolonged QTc intervals significantly associated with adverse clinical outcomes.9 In our cohort, the mean QTc in category C patients was 399.9 ±40.7 ms, however, this was not significantly associated with patient outcomes, likely due to the small number of patients in each category. While most studies reported non-specific abnormalities such as ST depression, T wave inversion, and QRS widening, our study found T wave inversions to be predominantly noted in category C patients (p=0.02), although these did not correlate with patient outcomes. In contrast to the frequent reports of RV strain and right axis deviation in the literature, our findings were markedly different, with left axis deviation being more prevalent (24.7% in category C) compared to right axis deviation (3.2% in category C). RV strain was observed in 7.2% of category C patients. Notably, P wave abnormalities, particularly left atrial abnormality (previously termed enlargement) and biphasic P waves, were more common. Other findings significantly associated with poor outcomes that echo broader research trends included the presence of left ventricular hypertrophy and poor R wave progression.
Despite providing insights into the cardiovascular dynamics of severely ill COVID-19 patients, this single-centre, prospective study’s limitations include its observational nature and limited sample size, which underscores the need for further research in a larger, more diverse population to generalise these findings.
Conclusion
Serial electrocardiograms offer essential insights into the cardiovascular haemodynamic of critically ill COVID-19 patients, which can help to categorise severity and predict outcomes.
Clinical Perspective
- Electrocardiograms could be used in serial monitoring of critically ill COVID-19 patients and to predict adverse outcomes.
- A new rS complex in lead III, with ST-T changes in the precordial leads, may indicate a sudden rise in left ventricular end-diastolic pressure and predict clinical worsening.
- Age and left atrial enlargement are independent predictors of adverse outcomes in people with severe COVID-19 infection.