Among patients with chest pain and normal coronary angiography, an unknown number have cardiac pain of ischaemic origin. However, normal epicardial coronary arteries have been found in approximately 20% of patients undergoing coronary angiography for chest pain evaluation.1 Over recent years, many terms have been proposed to describe chest pain due to myocardial ischaemia despite normal coronary angiography, including cardiac syndrome X, microvascular angina and non-atherosclerotic myocardial ischaemia. In 1973, Kemp et al. noted a heterogenous group presenting with typical angina and a positive exercise stress test but with normal epicardial coronaries on coronary angiogram.2 No single underlying mechanism has been identified for the condition. Vascular (endothelial and microvascular) dysfunction, coronary vasospasm and myocardial metabolic abnormalities have been implicated, although none of these factors is universally accepted. Patients with cardiac syndrome X, in whom chest pain may occur as a result of subendocardial ischaemia due to coronary microvascular dysfunction (CMD). This condition is frequently termed microvascular angina (MVA). In microvascular angina, the small calibre of those microvessels could not be evaluated by coronary angiography.3 The symptoms of patients with MVA are often indistinguishable from those of obstructive coronary artery disease (CAD). Patients with MVA frequently have diffuse subendocardial dysfunction. This underlying pathology could potentially affect ventricular longitudinal functions, but the degree of abnormality is often subtle. Thus, establishing a more sensitive index for early-stage left ventricular (LV) dysfunction is of great importance. In recent years, myocardial tissue deformation analysis by echocardiographic speckle-tracking imaging has provided new insights into cardiac function assessment. Global longitudinal strain (GLS), which can be detected by 2D speckle tracking echocardiography (2D-STE), is considered particularly sensitive for the evaluation of subendocardial ischaemia, haemodynamic overload or early myocardial damage at a stage when the LV ejection fraction (LVEF) is not yet impaired. Conventional echocardiography at rest provides little information regarding the presence of subclinical LV systolic dysfunction. There is growing evidence supporting the use of 2D strain in the identification of various levels of ischaemia and the ischaemic cascade that is responsible for significant reduction in longitudinal strain.
This study evaluated the diagnostic value of GLS as a non-invasive predictor for detecting subclinical LV systolic dysfunction in patients with angina pain with normal coronary arteries.
Study Objective
The objective of this study was to detect subclinical LV systolic dysfunction by GLS using 2D-STE in patients with angina and normal coronary arteries and normal baseline conventional echocardiograms.
Methods
Study Design and Population
In this comparative case–control study, patients with angina and normal epicardial coronary arteries who underwent coronary angiography in University Cardiac Centre, Bangabandhu Sheikh Mujib Medical University (Dhaka, Bangladesh), for evaluation of anginal pain fulfilling the inclusion and exclusion criteria were recruited as the study sample. Consecutive convenient (purposive) sampling was applied to select samples.
Cases
Male and female patients aged 18–55 years with angina and normal epicardial coronary arteries on coronary angiogram during evaluation for angina were included. Those with known ischaemic heart disease, myocardial scar or wall motion abnormalities on rest echocardiography, LVEF <55%, valvular heart diseases, pregnancy cardiomyopathies, abnormal coronaries on angiography (any vascular irregularity/ectasia/stenoses), hypertension, diabetes, severe anaemia or thyrotoxicosis, or aged <18 and >55 years were excluded.
Controls
In the control group, participants were prospectively recruited at a single tertiary centre, including hospital employees, fellows in training, their relatives, or individuals who underwent medical visits for driving or working licences who met the inclusion criteria. Male and female participants aged 18–55 years, with no history of cardiovascular or lung disease, no cardiovascular risk factors (i.e. hypertension, smoking, diabetes, dyslipidaemia) and normal results on physical examination and electrocardiography were included. Those with hypertensive heart disease with or without LV hypertrophy, valvular heart disease, idiopathic hypertrophic or dilated cardiomyopathy, acute or chronic inflammatory diseases, history of myocarditis and vasculitis, pregnancy and diseases of the aorta or arrhythmia were excluded.
Variables
Demographic, clinical and echocardiographic variables were as follows: age, sex and BMI; pulse rate, blood pressure (BP), Hb and ECG; and LVEF (%).
Analytical Procedures
Speckle-tracking imaging analysis was performed using commercially available software (EchoPAC BT12, GE Vingmed Ultrasound AS). The endocardial inner border of the LV was manually traced slightly inside the myocardium; a second, larger, concentric circle was then automatically generated near the epicardium to include all the LV myocardium. The software automatically divided each LV view into six equal segments and performed the speckle tracking on a frame-to-frame basis.
Normal epicardial coronary arteries were defined as having no visible disease or luminal irregularities (<50%) as judged visually at coronary angiography. Participants underwent 2D-STE for measurement of myocardial strain immediately before discharge. It was performed in the apical four-chamber, two-chamber and apical three-chamber views. Using speckle tracking, the endocardial border was traced in end systole. In cases of poor tracking, the region of interest was readjusted. The results of all three planes were combined in a single ‘bull’s eye’ summary (agreeing with the standard 17-segment model), which presents the analysis of each segment along with a global peak systolic strain value for the LV.
We defined the normal range of GLS using the receiver operating characteristic (ROC) with 95% CIs because normal values for the GLS analysis system are not well established in our country.
Statistical Analysis
Statistical analysis was performed using standard statistical software (SPSS Version 20). Continuous data are presented as mean ± standard deviation; categorical data are presented as frequencies and percentages. Between-group differences were evaluated using the unpaired Student’s t-test. The χ2 test was used for categorical variables and the unpaired t-test for continuous variables. A p-value of <0.05 was statistically significant.
Results
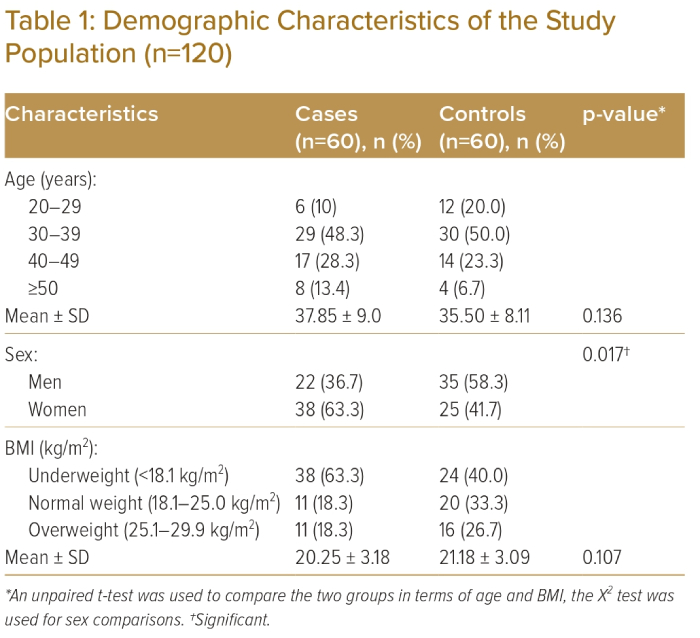
In both groups, the majority of participants were aged 30–39 years; 29 (48.3%) in the case group and 30 (50.0%) in the control group (Table 1). Mean age was 37.85 ± 9.0 years and 35.50 ± 8.11 years in the case and control groups, respectively (p>0.05). Thirty-eight (63.3%) participants and 25 (41.7%) were women in the case and control groups, respectively (p<0.05). Mean pulse rate was 82.03 ± 11.93 BPM, mean systolic BP was 124.33 ± 9.63 mmHg and mean diastolic BP was 77.33 ± 5.78 mmHg in the case group (Table 2).
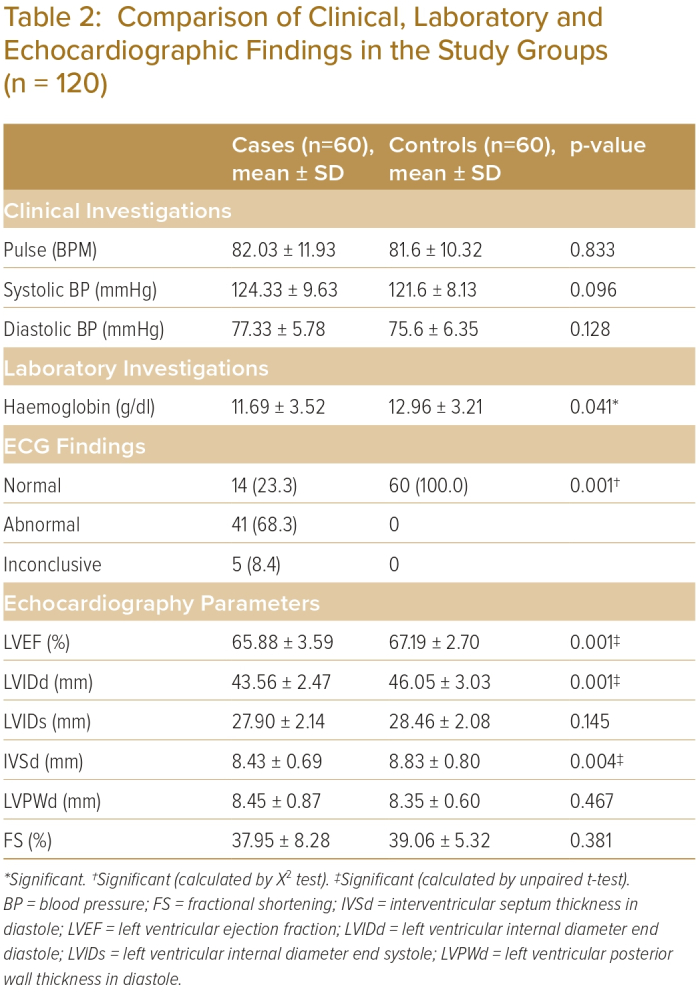
Fourteen (23.3%) participants in the case group and 60 (100.0%) in the control group had normal ECG findings (Table 2). An abnormal ECG was found in 41 (68.3%) of participants in the case group; there were no abnormal ECG findings in the control group. The ECG was inconclusive in five (8.4%) participants in the case group; there were no inconclusive ECG findings in the control group. The difference was statistically significant (p<0.05) between the two groups.
Mean LVEF was 65.88% ± 3.59% in the case group and 67.19% ± 2.70% in the control group (Table 2). In the case and control groups, respectively, mean LV internal diameter end diastole (LVIDd) was 43.56 ± 2.47 cm and 46.05 ± 3.03 cm; mean LV internal diameter in end systole (LVIDs) was 27.90 ± 2.14 cm and 28.46 ± 2.08 cm; mean interventricular septal wall thickness in diastole (IVSd) was 8.43 ± 0.69 cm and 8.83 ± 0.80 cm; mean LV posterior wall thickness in end diastole (LVPWd) was 8.45 ± 0.87 cm and 8.35 ± 0.60 cm; and mean fractional shortening (FS) was 37.65 ± 8.28 cm and 39.06 ± 5.32 cm. Mean LVEF, LVIDd and IVSd were statistically significantly different (p<0.05) between the two groups.
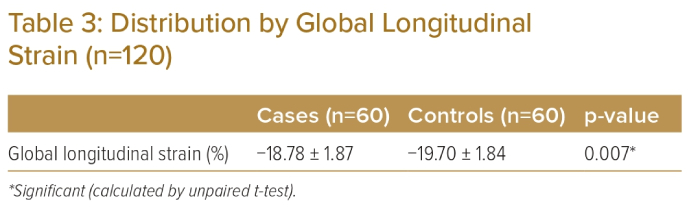
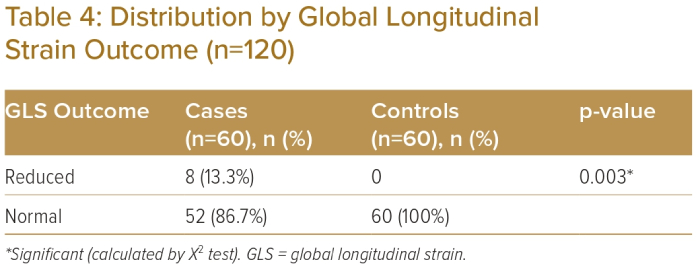
Mean GLS was −18.78 ± 1.87% in the case group and -19.70 ± 1.84% in the control group and was statistically significantly different between the groups (p<0.05; Table 3). Reduced GLS was observed in eight (13.3%) participants in the case group but was not observed in the control group (Table 4). Fifty-two (86.7%) participants in the case group and 60 (100%) in the control group had normal GLS. The difference was statistically significant between the two groups (p<0.05).
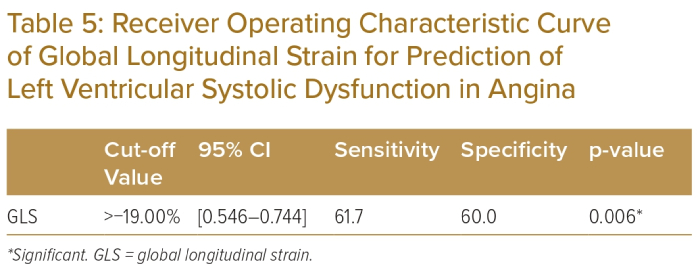
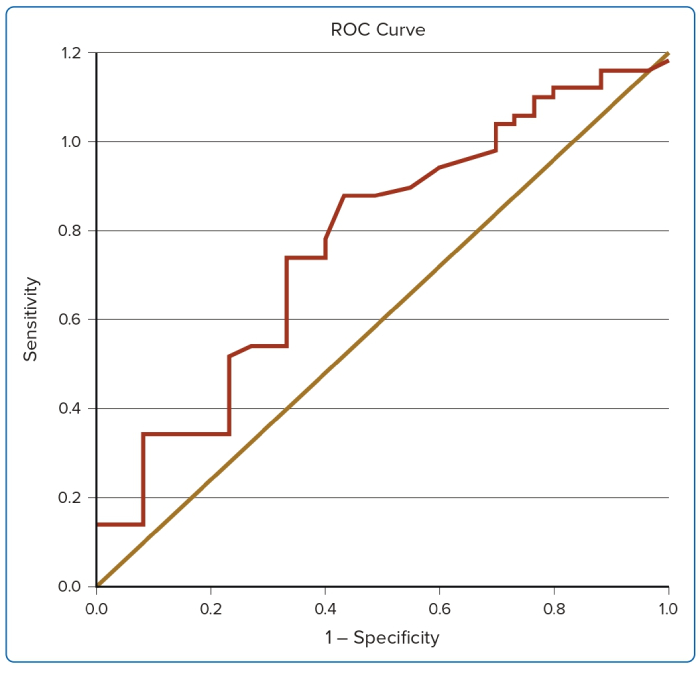
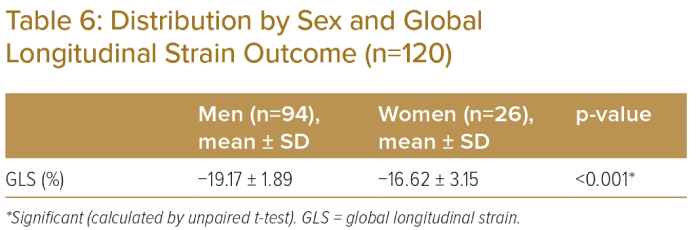
The area under the ROC curve for the prediction of LV systolic dysfunction is depicted in Table 5. GLS had an area under the curve of 0.625 (Figure 1). The ROC curve was constructed using GLS, which gave a cut-off value of >-19.0%, with 61.7% sensitivity and 60.0% specificity for the prediction of LV systolic dysfunction in angina. Eight participants had reduced GLS, among whom five (62.5%) were women and three (37.5%) were men. Mean GLS was -19.17 ± 1.89% in males and −16.62 ± 3.15% in females (Table 6; p<0.05).
Discussion
This study enrolled a total of 120 individuals (60 in the case group, mean age 37 ± 9.00 years; and 60 in the control group, mean age 35 ± 8.11 years; p>0.05). There was no statistically significant difference between the two groups regarding age, heart rate, systolic BP and BMI.
Among the whole studied sample, a significant positive correlation was evident between the participants’ symptoms and ECG findings; 68.3% of participants with typical chest pain with normal coronary angiogram exhibited ST-T abnormalities. On the contrary, nearly one-quarter (23.3%) of participants with typical chest pain had a normal ECG, whereas all (60) participants had normal ECG in the control group. Chowdhury et al. showed that approximately one-third (32.6%) of patients with normal coronary angiograms had normal ECGs.4 Concerning the LV characteristics of the study population, conventional echo parameters showed that there were statistically significant differences in the mean LVEF, LVIDd and LVSd between the two groups (p<0.05). The mean LVEF was 65.88% ± 3.59% in the case group and 67.19% ± 2.70% in the control group. Hb was 11.69 ± 3.52 in the case group and 12.96 ± 3.21 in the control group; it was significantly low in the case group (p<0.041).
This is similar to the Dillikar et al. study, in which the mean LVEF was 54% ± 9% in the CAD group and 56% ± 10% in the normal group.5 Dalen et al. observed that the mean overall GLS was −17.4%.6 Bansal and Kasliwal reported that the normal GLS was usually in the range of −16% to −18% or more (i.e. more negative).7 The present study showed that the mean longitudinal strain was significantly impaired in the case group relative to the control group (−18.78 ± 1.87 versus −19.7 ± 1.84%; p<0.007). This study also identified the normal reference value of the mean GLS in the healthy control group as −19.7 ± 1.84%.
ROC curves were constructed to determine the cut-off values for diagnosing GLS among the studied sample. Based on the ROC curve, GLS had an area under curve of 0.625, which gave a cut-off value of GLS >−19.0%, with 61.7% sensitivity and 60.0% specificity for the prediction of LV systolic dysfunction in the case group. Biering-Sørensen et al. demonstrated that the optimal cut-off value for GLS with the highest sensitivity and specificity for diagnosing CAD was −18.4%, with a sensitivity of 0.74 and a specificity of 0.58.8.
In this study, GLS was significantly attenuated (13.3%) in participants with typical chest pain despite normal LVEF and coronaries. Multiple pathophysiological abnormalities, such as microvascular disease, abnormal coronary vascular resistance and subendocardial ischaemia, have been reported to be responsible for the deterioration of LV longitudinal systolic function. A growing body of evidence suggests that MVA potentially affects ventricular longitudinal systolic functions. While evaluating the value of STE for detecting coronary microvascular disease, Galuszka et al.9 observed that GLS was significantly lower (GLS −15.8 versus −17.6; p<0.001) in patients with microvascular disease than in healthy controls.
The present study demonstrated a statistically significant difference in mean GLS between men and women (−19.17 ± 1.89% and −16.62 ± 3.15%). Emerging data support a sex-specific role of CMD. The combined presentation of angina and nonobstructive CAD increases risk of major adverse cardiovascular events.10 CMD is increasingly recognised as a potential cause of angina in these patients.11 Traditional cardiovascular risk factors are more common in women and many of these risk factors have either a greater impact or a higher prevalence – or both – in women. The metabolic syndrome (the combination of central obesity, glucose intolerance, hypertension and dyslipidaemia), is more common after menopause, which has the highest risk of developing CVD compared with both men with metabolic syndrome and those without this syndrome. According to the Women’s Ischaemia Syndrome Evaluation study, almost half of the women who had measures of coronary flow reserve had abnormal responses consistent with microvascular coronary dysfunction (MCD).12 This evidence of MCD may be a part of ischaemic heart disease pathophysiology and may explain the higher rates of angina in women.
The syndrome of angina or angina-like chest discomfort with normal findings on coronary arteriography is an important clinical entity. This syndrome was generally regarded as having a benign long-term prognosis but is now recognised to be associated with an increased risk for adverse outcomes in certain subsets of patients. These clinical subsets of patients are commonly included in microvascular disease in whom the small calibre of the vessels would be beyond negotiation of coronary angiography. Therefore, reduced GLS assessed by 2D-SE can identify the presence of microvascular disease in patients with typical chest pain with a normal coronary angiographic finding.
The study has some limitations. Firstly, the study population was selected from one selected hospital in Dhaka city, so the findings may not be generalisable to the wider population. Secondly, the small sample size was also a limitation.
Conclusion
Patients with chest pain without obstructive CAD are a heterogeneous group in which a significant number have experienced microvascular angina. While most CMD patients have a good prognosis, some have gradually aggravating angina symptoms that greatly affect the quality of life. Therefore, the diagnosis of MVA is important from both the therapeutic and prognostic perspective. However, there is no standardised non-invasive approach to defining CMD, making the evaluation of treatment strategies for MVA challenging.
Recently, PET and cardiovascular MRI have become more widely used to evaluate microvascular dysfunction in patients with chest pain without obstructive CAD. Nevertheless, even with these advances, the optimal strategy for diagnosing CMD is not clear. Coronary flow reserve measurements are performed only in highly specialised centres to diagnose microvascular disease. GLS assessment using speckle-tracking analysis of 2D echocardiography may offer a clinically feasible alternative to evaluate CMD. This study showed that GLS was significantly reduced in patients with CMD despite normal LVEF on conventional echocardiography, which may differentiate a microvascular group of patients from others who have angina with normal epicardial coronaries.
Most important modifiable cardiovascular risk factors, such as BMI, systolic and diastolic BP, high cholesterol and diabetes, have been reported to reduce LV longitudinal strain even in patients with preserved LVEF. In recent years, STE has been used to assess the GLS of the LV to detect subclinical systolic dysfunction. Furthermore, impaired GLS is an independent predictor of adverse cardiovascular events such as MI, heart failure, hospitalisation, and cardiovascular mortality. Thus, early detection of cardiac dysfunction using GLS and strain distribution may prove beneficial in the management of patients with microvascular disease. 
Clinical Perspective
- Patients with typical chest pain with normal epicardial coronary arteries may have underlying microvascular disease.
- Patients with microvascular disease have been a diagnostic and therapeutic challenge, contributing to significant economic, social, and healthcare costs.
- Global longitudinal strain may offer a safe, cost-effective routine non-invasive tool for the early detection of subclinical left ventricular systolic dysfunction in such patients.
- Microvascular dysfunction is an independent predictor of cardiovascular events and early detection may help identify appropriate candidates for further evaluation, aggressive risk factor modification and treatment.
- This study helped to establish normal values for the global longitudinal strain analysis system in Bangladesh, which has not been studied before.