AF is one of the most common cardiac arrhythmias.1 In Asia, the prevalence of AF ranges from 0.1% to 4% according to community-based studies and from 2.8% to 14% according to hospital-based studies.2 It is estimated that 72 million patients in Asia will have AF by 2050, of which 2.9 million patients will have strokes associated with AF.3 Stroke is the second leading cause of death worldwide and the third in developed countries, with ischaemic stroke accounting for 67–81% of all strokes.4 In the US, AF is attributed to 15–20% of all incidents of stroke. The mortality rate is higher for AF-related strokes than for any other type of stroke.
AF and valvular heart diseases often coexist.1 When they occur together, the risk of ischaemic stroke increases 17-fold.5,6 The incidence of valvular heart diseases in AF patients (valvular AF) is increasing globally, occurring in 2–31% of all AF cases.7 Cardioembolic stroke in AF cases primarily occurs due to blood stasis in the left atrium (LA). The LA appendage is the most common site for thrombus formation, accounting for 90% of thrombus in the LA.8 The risk of stroke is higher among patients with valvular AF than among those with non-valvular AF. It has been suggested that patients with valvular AF are at an elevated risk of thromboembolic events due to the presence of Virchow’s triad – venous stasis, vascular injury and hypercoagulability. These factors may be compounded by the presence of concurrent comorbidities.2,9,10 However, several studies have shown that the incidence of stroke varies even among this subgroup, with a reported 2.6% among 1-year anticoagulated valvular AF patients in Denmark, 4% among the valvular AF population in Turkey and up to 14.6% in a multicentre study conducted by Lip et al. in Europe.5,6,11
A recent consensus categorised AF patients with valvular heart diseases into two groups: evaluated heart valves, rheumatic or artificial (EHRA) type 1, which includes moderate-to-severe mitral stenosis or post-replacement mechanical prosthetic valves, and EHRA type 2, which includes native valve heart disease, mitral valve repair, bioprosthetic valve replacement and transaortic valve intervention (TAVI).1,2 Valvular AF is common in Asian and African populations, which may be due to the high incidence of rheumatic heart disease.
In Asia, the use of warfarin to prevent stroke in valvular AF patients has several problems. First, the optimal international normalised ratio (INR) for the usage of warfarin in Asia remains debatable, as ‘Asian’ ethnicity is very broad. Based on a nationwide registry, INR levels between 1.6 and 2.6 have been reported as optimal for preventing thromboembolism in Japanese patients with valvular AF, without increasing the risk of a major haemorrhage.12 Guidelines in Taiwan, Korea and China recommend an INR range of 2–3 as optimal for non-valvular AF patients treated with warfarin.13 Second, studies have reported that Asian patients face difficulties in maintaining therapeutic levels of INR (2–3) and have poor time in therapeutic range (TTR) values, which may be due to the high intake of certain herbal medicines or poor anticoagulation among patients in low- and middle-income countries in Asia.3,14 Third, the incidence of bleeding and haemorrhagic stroke is higher among Asian patients (0.75–1.33%) than among non-Asian patients (0.32–0.41%). The incidences of ischaemic stroke and systemic embolism have also increased due to most patients having an INR <2.3
CHA2DS2-VASC is a validated score that assesses stroke risk stratification and is commonly used for non-valvular AF.15 However, there is currently no risk stratification that specifically assesses the risk of stroke in the AF population with valvular heart diseases. Several studies, including Lip et al. in 2018, have validated the use of CHA2DS2-VASC for EHRA type 1 and 2 and showed that the tool’s predictive value in assessing the risk of stroke in this population is poor.5,6,16 At the time of the present study, there was no existing research on this subgroup in the Asian population. Therefore, we aimed to evaluate the clinical factors and echocardiographic parameters related to the incidence of ischaemic stroke in anticoagulated EHRA type 1 valvular AF patients.
Methods
This was a single-centre observational study involving patient data from the Indonesian Registry on Atrial Fibrillation (OneAF). A retrospective cohort study design was used to analyse the patients’ medical records. Type 1 EHRA valvular AF was defined as predominant moderate-to-severe mitral stenosis (which included pure mitral stenosis and mitral stenosis with mitral regurgitation, aortic valve stenosis and aortic valve regurgitation) and mechanical prosthetic valve replacement (which included: mechanical mitral valve replacement; mechanical mitral and aortic valve replacement; mechanical aortic valve replacement and mitral stenosis; and mechanical aortic valve replacement and mitral valve repair). The inclusion criteria for this study were as follows: patients with anticoagulated EHRA type 1 valvular AF in the OneAF registry between January 2015 and December 2019. The exclusion criteria were as follows: patent foramen ovale (PFO) identified from echocardiography results, ischaemic stroke without clinical or echocardiographic data in the year before incidence, periprocedural ischaemic stroke and missing data in the medical records. The baseline characteristics collected included medical history, AF type, symptoms and medications (vitamin K antagonist, antiplatelets and statins). Data regarding the components of the CHA2DS2-VASC score were also collected to assess stroke risk.
AF type was divided into two categories, which were paroxysmal AF and non-paroxysmal AF. Echocardiographic parameters were obtained from transthoracic echocardiograms. The stroke group’s laboratory data were taken for 1 year before the stroke event. TTR was calculated from the INR data using the Rosendaal method; the INR values ranged from 2.0 to 3.0. The CHA2DS2-VASC score was calculated for every patient. Based on the medical records, the clinical outcome was an ischaemic stroke that occurred during the monitoring period from January 2015 to December 2019. The patients were stratified into two distinct subgroups: those with a documented history of ischaemic stroke and those without.
This study was approved by the Indonesian Ministry of Health Research Ethics Committee (LB.02.01/2/KE.674/2020) and complied with the Declaration of Helsinki.
The statistical analysis was performed using statistical package for the social sciences (SPSS) software for Macintosh, version 26 (IBM). Categorical variables were presented in frequencies and proportions, while continuous variables were expressed as mean ± SD values or medians with ranges. The differences between the two groups were analysed using a t-test or the Mann–Whitney U-test for numeric variables and the χ2 test or Fisher’s exact test for discrete variables. Certain variables such as age, left ventricular ejection fraction (LVEF), tricuspid annular plane systolic excursion (TAPSE), creatinine, estimated glomerular filtration rate (eGFR), CHA2DS2-VASC score, LA diameter, LA volume index (LAVI), haemoglobin and TTR were categorised accordingly. Variables with p<0.25 in bivariate analyses were then included in a multivariate logistic regression analysis. A two-tailed p-value <0.05 was considered statistically significant.
Results
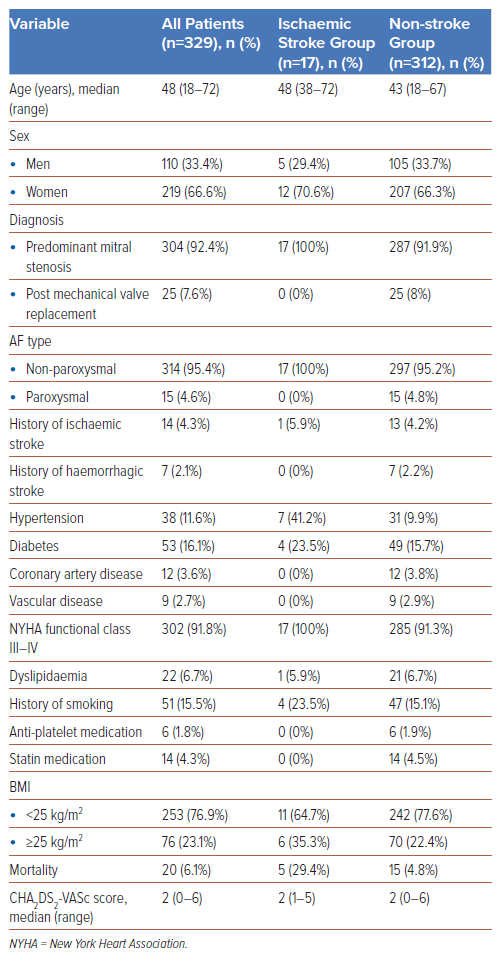
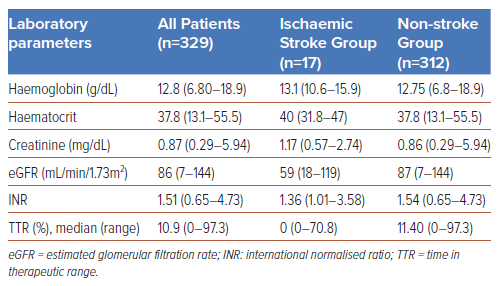
The baseline patient characteristics of two subgroups, ischaemic stroke and non-stroke, are summarised in Table 1, and the laboratory parameters are given in Table 2.
Most of the patients were women (66.6%), and most of the study population had isolated mitral stenosis (70.5%). A total of 302 (91.8%) subjects were categorised under the New York Heart Association (NYHA) functional class III–IV. Most patients (n=314, 95.4%) had non-paroxysmal AF. The median CHA2DS2-VASC score was 2, and the median LVEF (using the Teicholz formula) was 59%. The mortality rate was 6.1% (n=20), and the median TTR value was 10.9% for the entire population.
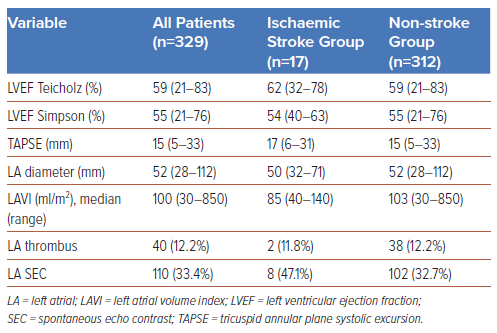
Among 329 AF patients with mitral stenosis or prosthetic mitral valves, the incidence of ischaemic stroke was 17 (5.2%). Hypertension (n=7, 41.2%), diabetes (n=4, 23.5%), and history of smoking (n=4, 23.5%) were more prevalent in the ischaemic stroke group than in the non-stroke group. The mortality rate was also higher for the ischaemic stroke group (n=5, 29.4%). The median CHA2DS2-VASC score for the two groups was 2. As for the echocardiographic parameters, the non-stroke group had a lower LA spontaneous echo contrast (SEC) percentage (32.7%) and a greater median LA volume index (103 ml/m2) than the ischaemic stroke group, as shown in Table 3.
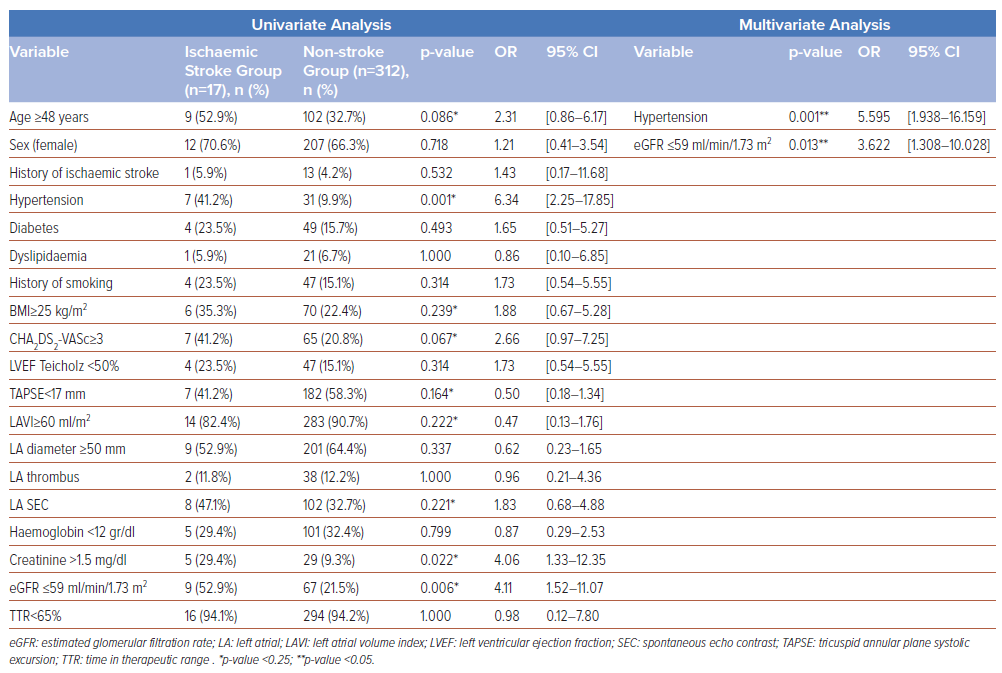
Based on the univariate analysis in Table 4, the following variables were included in the multivariate analysis: age >48 years (p=0.086); hypertension (p=0.001); BMI>25 kg/m2 (p=0.239); CHA2DS2-VASC score >3 (p=0.067); TAPSE <17 mm (p=0.164); LA volume index >60 ml/m2 (p=0.222); SEC at LA (p=0.221); serum creatinine >1.5 g/dl (p=0.022); eGFR <59 ml/min/1.73 m2 (p=0.006). We did not include the serum creatinine value in the multivariate analysis, despite it having a p-value <0.25, due to collinearity with eGFR (representing kidney failure).
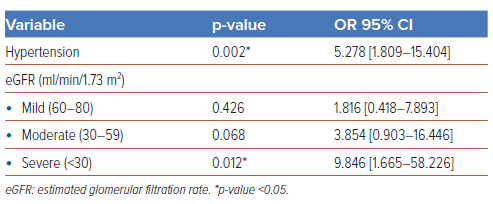
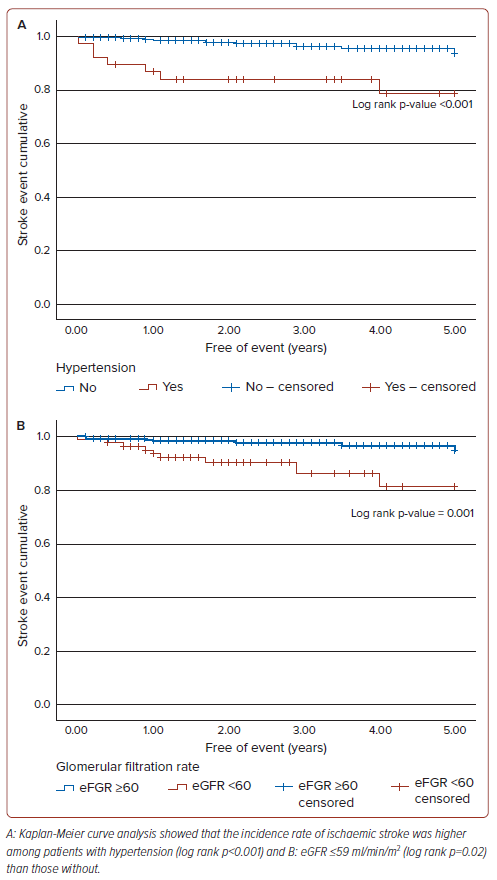
As shown in Table 4, the multivariate logistic regression analysis revealed that two clinical factors, namely hypertension (OR 5.59; 95% CI [1.93–16.15]; p=0.001) and eGFR ≤59 ml/min/m2 (OR 3.62; 95% CI [1.30–10.02]; p=0.013), were independently associated with the incidence of ischaemic stroke. A further stratification of kidney function showed that severe kidney failure (eGFR <30 ml/min/m) was independently associated with strokes in our patients (OR 9.85; 95% CI [1.67–58.27]; p=0.012), as shown in Table 5. No echocardiographic parameters were associated with the incidence of ischaemic stroke in the study population. A Kaplan-Meier analysis was performed using the two clinical factors, as shown in Figure 1. Within a 5-year period, the incidence rate of ischaemic stroke was higher among patients with either hypertension (log rank p<0.001) or eGFR ≤59 ml/min/1.73m2 (log rank p=0.02) than those without.
Discussion
We found that hypertension and abnormal renal function had statistical relationships with the incidence of ischaemic stroke in this study. Hypertension was found in 7 (41.2%) of the ischaemic stroke group and in 31 (9.9%) of the non-stroke group (p=0.001), a finding that is relevant to another report.17 While abnormal renal function (eGFR ≤59 ml/min/1.73m2) was observed in 9 (52.9%) of the ischaemic stroke group and 67 (21.5%) of the non-stroke group. A further classification of kidney function showed that severe kidney failure (eGFR <30 ml/min/1.73m2) was independently associated with strokes in our patients.
Ischaemic stroke has multiple aetiologies, which can be classified into three main categories: cardioembolic, large blood vessel atherothrombotic and lacunar stroke. Notably, AF is the most common cause of embolic thrombus in the brain. About one-third of stroke patients have undetermined aetiologies due to the presence of multiple aetiologies or a lack of solid evidence of a single cause of stroke.18 Atherosclerosis that occurs in the large extracerebral arteries, especially in the carotid bifurcation, is accelerated by hypertension. In addition, prolonged hypertension, particularly when suboptimally controlled, can lead to left ventricular hypertrophy, LA fibrosis/enlargement and diastolic dysfunction, increasing the risk of ischaemic stroke.19
As we only followed up on ischaemic stroke incidents retrospectively, we were unable to distinguish whether embolism or atherosclerosis was the underlying cause. Vascular changes resulting from hypertension or atherosclerosis could be responsible for ischaemic strokes in this demographic, in addition to embolism due to valvular AF. Our study did not include data on the patients’ carotid or vertebral artery conditions because vascular evaluations, such as active carotid artery evaluations, were not performed. In addition, no previous studies have specifically investigated the relationship between hypertension and the incidence of stroke in the same population as in the present study.
As mentioned earlier, eGFR ≤59 ml/min/1.73 m2 was the second clinical parameter that increased the incidence of ischaemic stroke in patients with type 1 EHRA valvular AF, along with a statistically significant increase in severe kidney failure (eGFR <30 ml/min/1.73 m2). No previous research has shown an association between eGFR and ischaemic stroke in this demographic, although similar results have been found in non-valvular AF populations. For instance, Vindhyal et al. showed that kidney failure, with an average eGFR value of 69.77 ml/min/1.73 m2, increased the CHA2DS2-VASC score.20 According to the findings of a study conducted in China, women with AF and impaired renal function are at a significantly higher risk of developing ischaemic stroke than their male counterparts. Further, a fivefold increase in the risk of stroke was reported for this subgroup of patients.21 This study predominantly featured women, a characteristic shared by our study. Chao et al. observed 547 non-valvular AF patients who underwent catheter ablation and found that impaired renal function (eGFR <60 ml/min/1.73 m2) increased the risk of thromboembolism despite ablation and also increased the accuracy of the CHA2DS2-VASC score.22 In AF patients with mild-to-moderate renal dysfunction, decreased renal function may increase the risk of stroke due to the activation of inflammatory and procoagulant pathways and changes in arterial compliance. In cases of end-stage renal failure after dialysis, conditions such as increased levels of endothelial-related factors and abnormal levels of coagulation and inflammatory factors lead to a prothrombotic environment.23
Currently, no ischaemic stroke prediction model that uses echocardiographic parameters has been applied to the same population as was the case in this study. The incidence of SEC in the LA was 47.1% in the ischaemic stroke group; however, its association with the incidence of stroke was not statistically significant (p=0.221). The median LA volume index was also high in the non-stroke group but was not statistically significant. The multivariate analysis in this study included two echocardiographic parameters: SEC and LAVI. However, the findings showed that neither of these parameters had a significant statistical relationship with the incidence of stroke in the study population.
The patients in this study were anticoagulated with warfarin. The efficacy of warfarin was analysed based on the INR and TTR values. For the stroke group, the median INR value was calculated using the overall INR value for a 1-year period prior to the stroke event, whereas for the non-stroke group, the overall INR value was calculated during the observation period. The Rosendaal method was used for the TTR calculations. The median INR values for the stroke and non-stroke groups were below the targeted values of 1.36 and 1.54, respectively (Table 2). TTR values ≥65% (based on the target INR of 2–3.0) were only found in 5.9% of the stroke group and in 5.8% of the non-stroke group. These findings suggest that more than 94% of our patients were undercoagulated. The low TTR levels may have been caused by the suboptimal administration of anticoagulants, patient non-adherence to medication and prolonged follow-up evaluations of INR.
Krittayaphong et al. considered data from a non-valvular AF registry in Thailand and found that patients with TTR ≥65% had a lower rates of ischaemic stroke, transient ischaemic attack (TIA), major haemorrhage, intracranial haemorrhage, and mortality than patients with TTR <65%.24 In Turkey, a study involving valvular and non-valvular AF populations had a median TTR value of 40%. Patients with TTR values ≥40% had lower rates of mortality, bleeding and hospitalisation than those with TTR <40%, but the risk of stroke did not differ between these two groups.11
In the present study, the TTR calculations were carried out with an INR limit of 2–3 in accordance with the European Society of Cardiology 2020 guidelines.25 The median TTR level in our study was only 10%, and it was 0% among patients with stroke events, lower than the levels reported in other published papers. Interestingly, the incidence of stroke was low in our patient population, even with TTR levels far below the target. Another factor that should be considered is that the appropriate target INR for the study population would likely be lower than the recommended limit (<2) to prevent thromboembolism. There was no evaluation of TTR at an INR level of 1.6–2.59, as was done in a Japanese study involving elderly patients with non-valvular AF.26 Nevertheless, analysing TTR and stroke in the present study was difficult, as over 94% of patients did not achieve the expected TTR levels. Further research involving a larger population in Indonesia is needed to confirm that optimal anticoagulation with a low median TTR does not increase the risk of stroke. Studies could also investigate the required INR target for effective thromboembolic prevention in this population.
The use of anticoagulants to treat AF patients with impaired renal function increased the risk of bleeding without decreasing the risk of thromboembolism, as shown by Shah et al. in a study conducted in Canada.27 Kidney disease may downregulate hepatic cytochrome CYP450 metabolism, leading to a dose adjustment for patients with moderate-to-severe chronic kidney disease.28 Periodic evaluations of the renal functions and anticoagulant doses for the type 1 EHRA valvular AF patients in this study should have been conducted due to the high risk of bleeding; however, this aspect was not evaluated.
Although all the data available on the type 1 EHRA valvular AF population in the OneAF registry were analysed in this study, the accuracy of the retrospective data was not as strong as those of prospective studies. It is worth noting that the study findings may have been affected by potential biases, as patients who passed away during the observation period were not excluded from the final analysis. This may have had an impact on the validity of the results and should be considered when interpreting the findings. We were unable to determine whether the ischaemic stroke events were caused entirely by embolisms arising from heart abnormalities, especially AF with valvular heart disease. Furthermore, this study did not involve the direct exclusion of patients with coagulation disorders, antiphospholipid syndrome, autoimmune diseases, a history of immobilisation, pregnancy or contraceptive use, which could have increased the risk of stroke.
Conclusion
We found that two clinical factors – hypertension and abnormal renal function – were markedly associated with stroke incidence among anticoagulated EHRA type 1 valvular AF patients. Therefore, it is necessary to control these comorbidities by controlling blood pressure and conducting periodic evaluations of renal function. A prospective cohort study is needed to confirm stroke events in EHRA type 1 valvular AF patients with hypertension and abnormal renal function. Further analysis is also required to determine more suitable tools for predicting the risk of ischaemic stroke in this population.
Clinical Perspective
- Our study shows hypertension and impaired renal function are associated with a higher risk of ischaemic stroke in valvular AF patients. This suggests that controlling blood pressure and monitoring and managing renal function in these patients may be a crucial preventive measure for reducing the risk of stroke.
- Our study highlights the need for developing more tailored scoring systems or risk assessment strategies for this specific patient group.
- Our study presents a potential avenue for future research and clinical guideline development in this field.