Historically, type 2 diabetes (T2D) has been associated with higher cardiovascular morbidity and mortality.1 Diastolic dysfunction (DD) is an early manifestation of diabetic cardiomyopathy and is linked to an increased risk of heart failure and mortality independent of systolic function decline.2 DD occurs early in diabetes, even when patients with diabetes are asymptomatic with normal blood pressure, normal contractility and no vascular complications.3–5
It is challenging to diagnose DD because evaluation of diastolic function (DF) using several parameters and different criteria is complex and the heterogeneity and ambiguity of different definitions from previous diagnostic algorithms results in significant variability in the reported prevalence and grading of DD.6–10 In 2016, a joint task force of the American Society of Echocardiography (ASE) and the European Association of Cardiovascular Imaging (EACVI) proposed a new and simplified algorithm for diagnosing and classifying DF.11
Several studies evaluated the impact of the 2016 recommendations compared to that of the 2009 recommendations and showed poor concordance and a significantly lower prevalence of DD when applying the 2016 recommendations.12–14 Nevertheless, outcome-based studies are lacking to demonstrate the prognostic implications of such comparisons in predicting outcomes in the T2D population.
In the present study, we prospectively evaluated the impact of the 2016 ASE/EACVI recommendations on estimates of DD, concordance between 2009 and 2016 recommendations in the diagnosis of DD and the prognostic accuracy of the 2016 algorithm in predicting cardiovascular outcomes in patients with hypertension and T2D but without a history of cardiovascular disease.
Methods
Study Population
This prospective cohort study included adults aged 18–65 years with T2D who were treated for at least 3 years. Individuals with known cardiovascular disease – defined as a history of MI, percutaneous coronary revascularisation, coronary artery bypass graft surgery, at least moderate valvular heart disease, bundle branch block, AF or atrial flutter, permanent pacemaker, arrhythmia, heart failure hospitalisation, or reduced left ventricular (LV) ejection fraction (<55%) – were excluded.
In addition, individuals with renal impairment (defined as an estimated glomerular filtration rate of <30 ml/min/1.73 m2), known history of bronchial asthma, pancreatitis, underlying endocrine disorders (such as acromegaly, Cushing’s syndrome, hyperthyroidism, pheochromocytoma and aldosteronism), uncontrolled hypertension (defined as systolic blood pressure of ≥200 mmHg, diastolic blood pressure of ≥120 mmHg or use of more than three antihypertensive drugs), life-threatening conditions with <1 year of life expectancy and poor echocardiographic windows were excluded.
All included participants underwent physical examinations and evaluation of cardiovascular risk factors and detailed anthropometric parameters. Blood samples were collected for evaluation of fasting lipid profiles, HbA1c levels, and renal profiles. All participants underwent comprehensive transthoracic echocardiography examinations at baseline and were followed up for 1 year to assess clinical outcomes. Data on clinical outcomes measures, a composite of death, MI, heart failure, stroke, AF or atrial flutter and hospital admissions for adverse cardiovascular events, were collected.
This study was performed in accordance with the principles of the Declaration of Helsinki. Ethical approval for this study was obtained from the Medical Research and Ethics Committee, Ministry of Health Malaysia (Research ID: NMRR-16-436-29619). All participants provided written informed consent before enrolment in the study.
Echocardiography
Transthoracic echocardiography examinations were performed using the Philips CX50 System (Philips Medical Systems) equipped with S3 probes (2–4 MHz). Stored images were analysed offline by an echocardiographic technician blinded to the clinical data. Three readings were obtained for each echocardiographic variable and averaged over three consecutive cardiac cycles. Chamber quantification and function were assessed based on the most recent 2015 ASE/EACVI recommendations.15 Mitral inflow velocities (E-wave and A-wave), E/A ratio, deceleration time (DT), tissue Doppler imaging septal and lateral e’ and a’ diastolic peak velocities, E/e’ ratio and tricuspid regurgitation (TR) peak velocity were obtained for diastolic function assessment.
Classification of DF
Both 2009 ASE/European Association of Echocardiography (EAE; now the EACVI) and 2016 ASE/EACVI recommendations were applied to determine LV DF and grades.6,11 According to the 2009 ASE/EAE recommendations, the determination of DF consisted of two steps. The first step started with the septal and lateral e’ velocities and the indexed left atrial (LA) volume.6 Two of three measures exceeding the cut-off value suggested were required to proceed to the subsequent step in grading of DD. The second step involved the assessment of E/A ratio, DT, and average E/e’ ratio. At least two positive measures were required to attribute the DD grade. DF was considered indeterminate it was not possible to grade DD due to discrepancies in measurements between these parameters.
In the 2016 ASE/EACVI recommendations, DF was evaluated using four variables: septal or lateral e’ velocity, indexed LA volume, average E/e’ ratio, and TR peak velocity.11 Based on the measurements obtained from all four variables suggested in 2016 recommendations, participants were classified as having normal DF (less than two of four positive criteria), DD (more than two of four positive criteria), or indeterminate DF (two of four positive criteria).
DF classification was performed by two independent investigators blinded to participant characteristics (MI and MNABS). All findings were then validated by a board-certified cardiologist with expertise in echocardiography (KHL), who reviewed all echocardiographic data and images acquired and was blinded to the clinical data of each participant.
Statistical Methods
Clinical characteristics and echocardiographic variables are presented as mean ± SD, and DF categories are presented as absolute numbers and percentages. Concordance between the 2009 and 2016 classifications was determined using the κ coefficient and the proportion of agreement. Concordance was defined as poor (0–0.20), fair (0.21–0.40), moderate (0.41–0.60), good (0.61–0.80), and optimal (0.81–1).16 The reclassification rate was calculated using the formula: 100% − proportion of agreement (%). Statistical significance was set at p<0.05. All data analyses were performed using SPSS software (IBM SPSS Statistics for Windows, Version 21.0).
Results
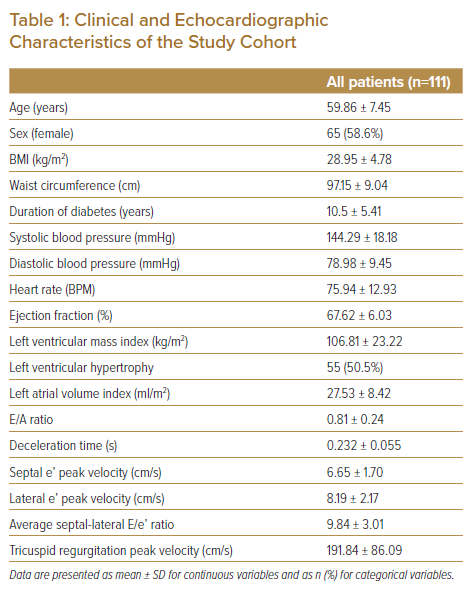
A total of 324 individuals with T2D and hypertension who attended the diabetes outpatient clinic follow-up in primary healthcare settings were screened for eligibility. Among the screened individuals, 111 who met the inclusion and exclusion criteria were enrolled. Table 1 shows the clinical and echocardiographic characteristics of the study cohort. There were 65 (58.6%) women. The mean age was 59.86 ± 7.45 years, and the mean duration of diabetes was 10.5 ± 5.41 years. Most patients were obese (BMI >25 kg/m2 and waist circumference >90 cm). The mean systolic blood pressure was elevated at 144.29 ± 18.18 mmHg. Echocardiography revealed LV hypertrophy in 50.5% patients.
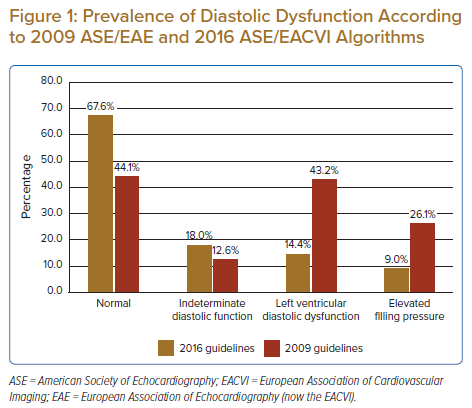
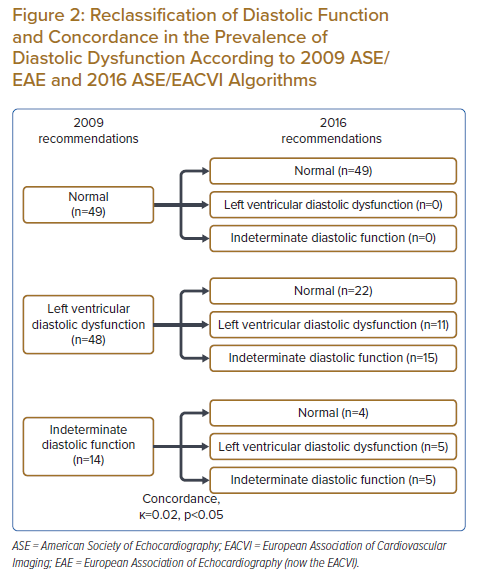
The prevalence of DD was much lower with the 2016 algorithm than with the 2009 algorithm (14.4% [n=16] versus 43.2% [n=48]; Figure 1). However, the prevalence of indeterminate (18.0% [n=20] versus 12.6% [n=14]) and normal DF (67.6% [n=75] versus 44.1% [n=49]) were higher with 2016 recommendations than with 2009 recommendations. Concordance between 2016 and 2009 recommendations was poor (k=0.02, p<0.05), with a reclassification rate of 41.4% (Figure 2). Among the 48 patients classified as having DD according to 2009 recommendations, 22 (46%) were reclassified as having normal DF and 15 (31%) were reclassified as having indeterminate DF according to 2016 recommendations. Nevertheless, there was concordance in 11 (23%) participants classified as having DD according to the 2016 and 2009 guidelines. Among the 20 patients classified as having indeterminate DF according to 2016 recommendations, 15 (75%) were reclassified as having DD according to 2009 recommendations. There was agreement only in five (25%) patients classified as having indeterminate DF according to both guidelines.
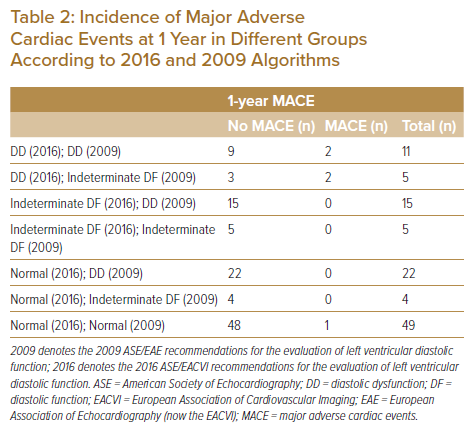
Based on the prevalence of DD determined according to previous and current recommendations, the impact of the application of the current 2016 algorithm was evaluated by assessing the incidence of major adverse cardiac events (MACE) at 1 year. As demonstrated in Table 2, none of the patients classified as having indeterminate and normal DF using the 2016 algorithm and DD and indeterminate DF using the 2009 algorithm developed MACE at 1 year. Among the 16 patients classified as having DD according to the 2016 algorithm, four (25%) developed MACE at 1 year; among them, two were also classified as having DD according to the 2009 algorithm, and the other two were classified as having indeterminate DF according to the 2009 algorithm.
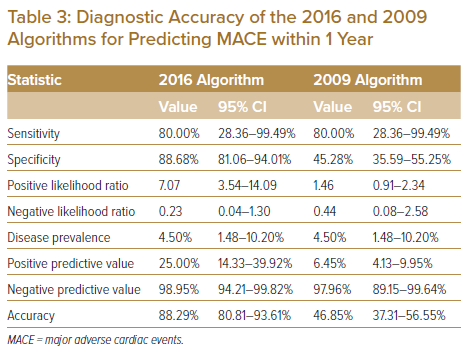
Supplementary Material Table 1 shows components of MACE developed at 1 year in our study cohort. Among the 48 patients classified as having DD based on the 2009 algorithm, only 2 (4.2%) developed MACE at 1 year. A comparison of the diagnostic accuracy of the 2016 ASE/EACVI and 2009 ASE/EAE algorithms is summarised in Table 3. The 2016 algorithm showed better diagnostic accuracy (88.29%, sensitivity 80.00%, specificity 88.68%, positive predictive value 25.00%, negative predictive value 98.95%) than the 2009 algorithm (46.85%, sensitivity 80.00%, specificity 45.28%, positive predictive value 6.45%, negative predictive value 97.96%) for predicting MACE at 1 year.
Discussion
DD is an important precursor of heart failure with preserved ejection fraction (HFpEF). A study from the ASIAN-HF registry found that many patients in southeast Asia who had HFpEF had associated diabetes.17 For example, in Malaysia, one-third of patients admitted for HFpEF had underlying T2D.18 These patients were found to have the worst, if not, the second worst outcomes, with a high rate of heart failure hospitalisation and mortality events within 1 year.17
The 2021 European Society of Cardiology guidelines for the diagnosis and treatment of acute and chronic heart failure have also included echocardiographic LV DD/raised LV filling pressure as an objective evaluation of cardiac functional abnormalities to diagnose HFpEF.19 Furthermore, the results of the EMPEROR-PRESERVED study have demonstrated benefits of sodium-glucose cotransporter 2 inhibitors (SGLT2I) − as a treatment option − in improving outcomes and lowering the risk of composite cardiovascular death and heart failure hospitalisation in patients with HFpEF.20 Hence, in practice, DD characteristics on echocardiography similar to those of HFpEF in patients with T2D should prompt clinicians to intervene early while patients are still asymptomatic for primary prevention to reduce the burden of the disease.
Early screening of patients who are at risk of developing adverse cardiac events and aggressive management of risk factors have been shown to reduce the rate of LV dysfunction and heart failure progression and improve clinical outcomes.21,22 In the context of local settings, this could prevent at least one-third of HFpEF hospitalisations.
This study is the first to assess the implications of the 2016 DF recommendations compared to those the 2009 DF recommendations in predicting cardiovascular outcomes in patients with T2D without a history of cardiac disease. The study cohort involved, i.e. patients with both T2D and hypertension, represented majority of our T2D population. Nevertheless, we believe that the inflammatory process of diabetes was the driver for the diastolic function in our study population as DD is usually associated with uncontrolled hypertension (which was an exclusion criterion in this study) because of pressure overload. Consistent with the findings of previous studies, our study showed that application of the 2016 ASE/EACVI DF criteria reduced the prevalence of DD significantly because of the use of a strict definition of DD (more than two of four positive criteria).12–14 According to our observation, the 2016 DF algorithm detected more advanced cases and performed better in terms of diagnostic accuracy than the previous 2009 algorithm. Nevertheless, although the current 2016 DF recommendations simplify the diagnosis of DD, they resulted in a higher number of indeterminate DF cases in our study cohort. It was observed that 75% of the indeterminate DF cases classified using the 2016 algorithm were reclassified as DD cases using the 2009 algorithm. Although none of the patients developed MACE, the 1-year follow-up period was very short to deduce whether patients who met two of four positive criteria could be considered as having normal DF. These patients did not fulfil sufficient criteria to be diagnosed with DD. Further investigations and closer monitoring are warranted to exclude DD, especially among patients with T2D and a dilated LA. An increased LA volume index has been associated with worse outcomes in patients with T2D and heart failure.23–27
The implication of this study is important as it is only the tip of the iceberg of cardiovascular risk prediction in diabetes. Indeed, a longer follow-up period should be considered in future studies. However, titration of treatments, which can change the natural course of T2D and hypertension, usually occur while managing patients with T2D at diabetes outpatient clinics in primary care settings. Therefore, the LV should be assessed by echocardiography every time HbA1c is measured as routine assessment of diabetes control as per recommendations for patients with diabetes.28 Then, from trajectory analysis of serial data obtained at different time points over a longer follow-up period, rather than distinguishing between normal and abnormal diastolic function, a structured outcome-driven predictive model with age- and sex-specific reference frames could be generated to better risk stratify asymptomatic patients with T2D. The resultant model will be more clinically relevant to the Asian population, which has a higher prevalence of T2D; younger age at heart failure presentation; higher association with HFpEF than with heart failure with reduced ejection fraction; and unique female heart failure characteristics associated with lean diabetic phenotype, higher prevalence of concentric LV hypertrophy , and worse outcomes.29–33,17 This will be parallel with recent changes introduced in clinical practice when therapeutic options – such as SGLT2Is that reduce risk of cardiovascular deaths or hospitalisations for heart failure − become available for patients with HFpEF, with or without diabetes.19,20
Study Limitations
This study has some limitations. First, this is a single-centre study involving a local Southeast Asian population. Thus, there is limitation with regard to generalisability to other populations particularly in the context of a heterogenous Asian population accompanied with geographical variances. Second, it included only asymptomatic patients with diabetes and LV ejection fraction of >55%. Therefore, the observations cannot be extrapolated to patients with diabetes and reduced LV ejection fraction on echocardiography. Third, pulmonary vein flow parameters, which are included in the 2009 ASE/EAE algorithm for the evaluation of LV DF,6 were not included in LV DF assessment in this study. However, this is not expected to significantly change the reported results. Finally, the duration of follow-up was very short to demonstrate the role of the current 2016 ASE/EACVI recommendations in distinguishing patients with T2D and hypertension who are at risk of developing MACE. A longer-term follow-up of a larger sample of the population with T2D involving serial investigations will be considered in future studies.
Conclusion
The application of the new 2016 recommendations resulted in a much lower prevalence of DD in this cohort. The updated criteria detect more advanced cases and predict short-term 1-year cardiovascular outcomes. Further studies are warranted to investigate the prognostic impact of these criteria.
Click here to view Supplementary Material.
Clinical Perspective
- This study is the first to compare 2016 versus 2009 diastolic function recommendations in cardiovascular risk prediction in patients with type 2 diabetes.
- The 2016 algorithm showed better accuracy than the 2009 algorithm in predicting major adverse cardiac events at 1 year.
- Left ventricular assessment by echocardiography may be recommended when the HbA1c level is measured if titration of treatments is involved.
- The implication of this study is important to enhance future study designs to adopt a more comprehensive approach involving the collection of clinical and imaging data and a longer follow-up period to better stratify cardiovascular risk in patients with type 2 diabetes, particularly when treatment options that improve outcomes in patients with diabetes and diastolic heart failure become available.