Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has infected nearly 600 million people, resulting in >6 million deaths, with high mortality rates from cardiovascular complications.1 Although initially known to primarily affect the pulmonary system, advancing literature has produced multiple theories of a more multi-systemic involvement, including the cardiac system. Several mechanisms have been proposed as the cause of cardiac damage: systemic inflammation due to cytokine storm, direct viral injury causing myocarditis, stress-induced cardiomyopathy and endothelial damage leading to microvascular thrombosis.2 Myocardial injury as a result of COVID-19 can lead to serious complications, such as acute and/or chronic heart failure, life-threatening arrhythmias and sudden cardiac death.3
A study by Lindner et al. demonstrated the presence of SARS-CoV-2 viral particles in the heart in 24 of 39 (58%) autopsies.4 Pellegrini et al. performed autopsies in 40 subjects and found that myocardial necrosis was present in 35% of subjects, of whom 78% (11/14) had microvascular and 21% (3/14) macrovascular thrombosis.5 Clinical indicators of myocardial injury include elevated cardiac markers such as troponin, electrocardiographic changes and reduced ejection fraction (EF).6 However, these tests may not provide adequate evidence for cardiovascular sequelae, especially in patients who had mild or moderate cases of COVID-19.
Current literature has demonstrated changes in cardiac troponin (cTn) levels, defined as an elevation above the 99th percentile upper reference limit at any time during admission, as a clinical indicator of myocardial injury. Elevated cTn was associated with a higher risk of adverse events, including mortality rate, hospitalisation and malignant arrhythmias.7–9 A study by Huang et al. reported that 12–15% of patients with COVID-19 had elevated cTn during their hospital stay and that cardiac involvement was predominantly observed in up to 31% of severe cases of COVID-19.10 There are a few studies illustrating complications, especially in patients with pre-existing cardiac conditions, where COVID-19 led to exacerbation of heart failure, development of long-standing malignant arrhythmias or pericarditis.11–13 In the study by Ruan et al., the development of fulminant myocarditis was observed in 7% of patients with fatal outcomes.14
Given the high rate of cardiac involvement in COVID-19 patients, there is still concern regarding whether this myocardial injury is sustained after recovery from SARS-CoV-2 infection. Despite evidence of biomarker determination and interpretation, this may not provide a clear illustration of cardiac complications as they only reflect severity of the SARS-CoV-2 infection rather than any certain pathology.15 Cardiac MRI (CMR) can be used to further evaluate cardiac morphology and function and may serve as a sensitive tool to identify myocardial oedemas and necrosis non-invasively and assess the severity of tissue damage. CMR is a potentially valuable diagnostic tool in patients with COVID-19 presenting with myocardial injury and evidence of cardiac dysfunction.16 Cardiac involvement in patients with COVID-19 is associated with adverse prognosis, and thus early identification is of importance. The challenge to characterise further tissue and myocardial changes brought about by COVID-19 can be accomplished using more advanced techniques, particularly CMR.
The aim of this study was to determine the incidence of myocarditis among patients who recovered from COVID-19 and underwent CMR. Baseline characteristics, including demographic profile, co-morbidities, and clinical and laboratory parameters, were recorded. We identified patients with positive conventional findings based on the presence of myocardial oedema and/or late gadolinium enhancement (LGE). We then compared these patients with those who had negative findings, focusing on ventricular functional parameters.10
Methods
The study was conducted at St Luke’s Medical Center, Quezon City, the Philippines, from January 2020 to December 2021. This was a single-centre, observational, retrospective cohort study of 54 adult patients with a history of a previously confirmed diagnosis of COVID-19 (through reverse transcription–polymerase chain reaction on nasopharyngeal/oropharyngeal swabs) and who were previously hospitalised for treatment of COVID-19. All patients included had received full vaccination (two doses) before their first COVID-19 diagnosis. Patients were eligible if they eventually recovered based on the WHO criteria.17 The index date for patients who recovered from infection was defined as the first day of negative test upon repeat reverse transcription–polymerase chain reaction performed after a complete course of therapy. Based on the protocol of our institute, patients had a follow-up after 3 months post-recovery and subsequently underwent CMR. Patients with the following conditions were excluded from the study: a history of coronary artery disease or previous cardiac surgery, acute coronary syndrome, a history of myocarditis before recovered COVID-19, general contraindications to CMR, unstable clinical condition or inability to perform repeated breath-holds. Written informed consent was obtained from all patients. The study was performed in accordance with the Helsinki Declaration and was approved by the Institutional Ethics Review Committee of the Research and Biotechnology at St Luke’s Medical Center, Quezon City, the Philippines.
Cardiac MRI Protocol
All patients included in the study underwent CMR with tissue characterisation on a 3T magnetic resonance scanner (3.0T Signa Pioneer Wide Bore MRI, GE Healthcare) using standardised protocols that included the following: conventional sequences: short-axis and long-axis cine, T2-weighted imaging (T2WI) and LGE; and early gadolinium enhancement. The images were assessed both visually and by using automated methods. Conventional sequences were used to acquire information on left and right ventricular structure, volume, mass and function, tissue characterisation, early gadolinium enhancement and LGE. The following CMR parameters (for both left and right ventricles) were collected: end-diastolic volume (EDV), EDV/body surface area (BSA), end-systolic volume (ESV), ESV/BSA, stroke volume (SV), SV/BSA, EF and T2WI assessing for myocardial oedema/inflammation and symptoms showing with LGE.
Image Analysis
One cardiologist (CMP, specialised in multimodality imaging) evaluated all CMR images using Vue Motion version 12.1.5.4 (Carestream Health). Myocardial oedema was evaluated using T2WI images and divided into 16 segments based on the American Heart Association guidelines.18 The location and pattern based on the LGE images were assessed and reviewed by two researchers independently (MC and SAP). A senior researcher (RET) adjudicated any discrepancies between the two observers. Patients involved in the study were divided into two groups based on the presence or absence of positive conventional CMR findings, defined as the presence of myocardial oedema and/or symptoms showing with LGE. The left and right ventricular function parameters were determined, including EF, EDV, ESV and SV. All volumes and masses were normalised using BSA.
Operational Definitions
The following definitions were used:
- Positive conventional CMR findings: defined as the presence of myocardial oedema and/or symptoms showing with LGE.
- Negative conventional CMR findings: defined as the absence of myocardial oedema and/or symptoms showing with LGE.
- Mild COVID-19: patients diagnosed with COVID-19 who were admitted to a regular ward without non-invasive respiratory or inotropic support.
- Moderate COVID-19: patients diagnosed with COVID-19 who were admitted to a specialist respiratory unit requiring non-invasive respiratory support.
- Severe COVID-19: patients diagnosed with COVID-19 who were admitted to the intensive care unit for closer monitoring.
Statistical Analysis
All statistical analyses were performed using SPSS v27 (IBM). Descriptive statistics were generated for all variables. Categorical data are summarised as frequencies and percentages, while continuous variables are expressed as mean and SD. The comparison between two groups was performed using unpaired Student’s t-test (for normal distribution) or Mann–Whitney U-test (for non-normal distribution) for continuous variables, or χ2 test for categorical variables.
Results
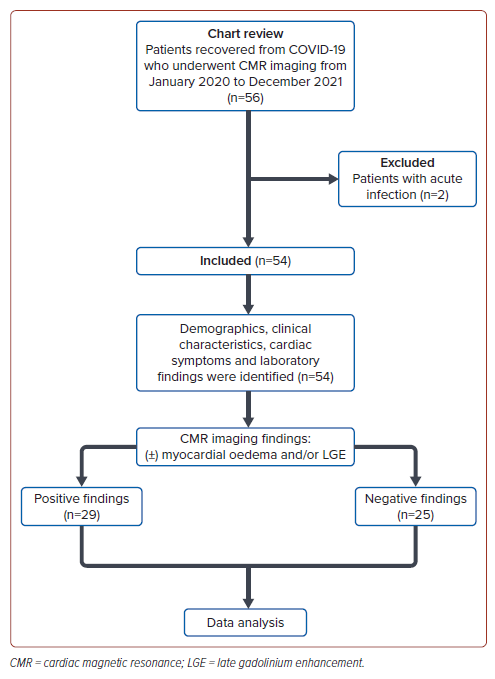
A total of 56 patients met the inclusion criteria. However, two patients were excluded because of the presence of acute infection (bacterial pneumonia) at the time of CMR. Thus, a total of 54 patients were included in the study. The flow diagram of the study is shown in Figure 1. There were 29 patients (54%) found to have positive conventional CMR findings indicative of myocarditis (myocardial oedema and/or LGE presence), while 25 (46%) had negative conventional MRI findings.
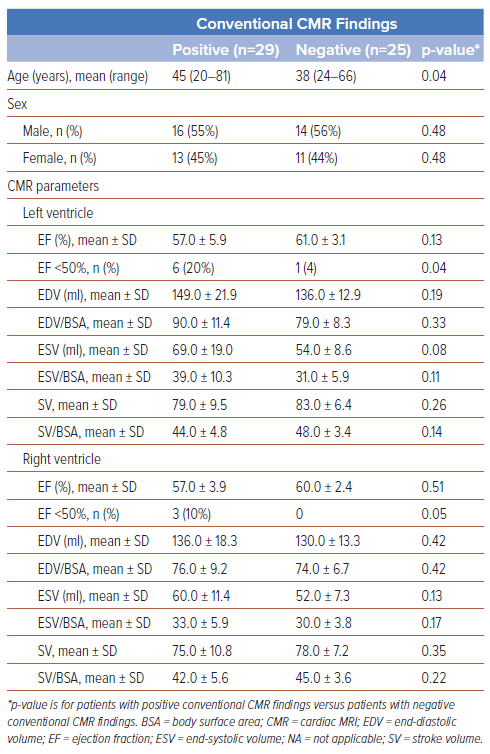
The demographics and clinical characteristics are presented in Table 1. The median age of patients was 41 years (range 20–81 years), and 55% of patients were male. Among all recovered COVID-19 patients with positive CMR findings (n=29), the mean BMI was 27.12 kg/m2 and average BSA was 1.79 m2. Thirty-one (57%) patients were diagnosed as having mild-type COVID-19, 14 (26%) patients as having moderate-type COVID-19 and 9 (17%) patients as having severe COVID-19. Mild COVID-19 was most commonly observed in recovered COVID-19 patients with positive CMR findings (n=17 [59%]).
Discussion
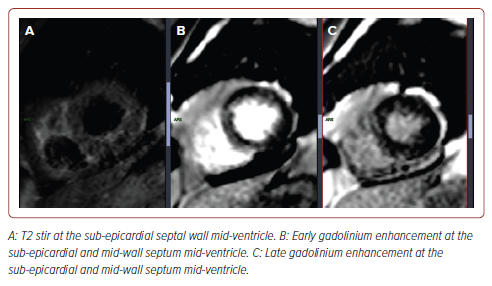
The findings show that 29 of 54 (54%) recovered COVID-19 patients presenting with cardiac symptoms had positive conventional CMR findings indicative of myocarditis. It is to be noted that all 54 patients had no previous myocarditis or heart disease. These findings suggest that COVID-19 may have lasting consequences in terms of cardiac involvement. A sample representative image from our study is shown in Figure 2.
Twenty-five of 54 patients reported cardiac symptoms such as palpitations, dyspnoea and chest discomfort. Only a few studies have identified persistent or new-onset cardiac involvement among recovered COVID-19 patients who are still experiencing COVID-19 symptoms. Huang et al. reported that 15 of 26 (58%) recovered COVID-19 patients who reported cardiac symptoms had abnormal CMR findings manifesting as myocardial oedema and fibrosis.10 Among the patients who reported chest discomfort, there was a notable difference between those who had positive findings on CMR imaging and those who had negative findings. This suggests that the presence or absence of certain abnormalities detected by CMR may be linked to the experience of chest discomfort. This could be attributed to the residual pulmonary effect of the disease or the timing of CMR examination from the onset of symptoms, which failed to reveal the presence of residual or ongoing inflammation.10
Among the 29 patients positive for CMR findings, 19 had previously undergone echocardiography during the time of COVID-19. Three (15%) had abnormal wall motion and contractility along with findings of global hypokinesia with the EF ranging from 14.2% to 25.4% by Simpson’s method. Post-recovery CMR findings showed improvement in EF as these patients were already being treated with heart failure medications (β-blockers, sacubitril/valsartan, diuretics, sodium-glucose cotransporter-2 inhibitors). The use of echocardiography in recovered COVID-19 patients is undoubtedly similar to that of CMR in terms of evaluating and quantifying global and regional systolic function. However, CMR may play an important role in therapeutic decision-making and has prognostic value because determination of myocardial oedema, scarring and fibrosis based on LGE is associated with a higher risk of adverse cardiovascular events.19 Furthermore, there was improvement in EF and wall motion abnormality among the three patients who previously had a low EF. Florian et al. and Huang et al. suggest that structural remodelling may occur before functional remodelling, which may be similar to our results because most of our abnormal findings were on myocardial tissue despite normal ventricular function.10,20
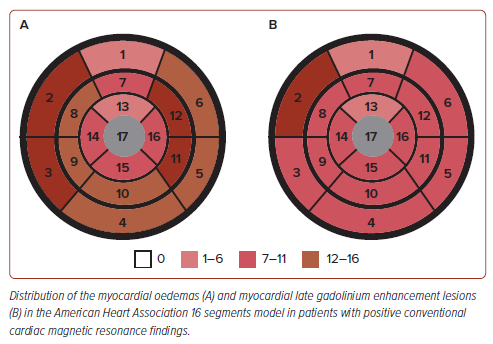
The presence of focal lesions detected using LGE and myocardial oedema was the major CMR finding in this study. The presence of lesions detected using LGE was detected in 26 (48%) patients who underwent CMR, with the most common lesions seen in the lateral walls with sub-epicardial and mid-wall involvement located in either the basal, middle and/or apical segments, followed by septal walls at the base to mid-chamber. Based on the segments identified in this study (Figure 3), the location of SARS-CoV-2 infection appears to be relatively similar to that of acute myocarditis, which commonly involves the inferior and inferolateral walls. Myocardial oedema was found in 31% (9 of 29) of patients. These results suggest that diffuse myocardial inflammation and fibrosis can occur in patients who recovered from COVID-19.
Three of 29 (10%) patients with positive CMR findings were found to have impaired right ventricular EF (<50%). Based on previous studies, this might be due to acute lung injury developing acute respiratory distress syndrome affecting the right ventricular function as it is easily affected by pulmonary vascular resistance.21, 22
Limitations
There are several major limitations in the study. This study focused on determining the cardiac involvement in patients who recovered from COVID-19 and underwent CMR for evaluation to rule out myocarditis in our institution from January 2020 to December 2021.
Patients included in the study may have completed medical management for their COVID-19 and are considered to have recovered by their respective physicians. The vaccines received by the patients were not similar, although there were no reported adverse events that may be secondary to vaccinations. The specific duration of CMR examination from the time of recovery was not consistent, and some patients may have undergone the test at different times. Nonetheless, we set the duration of follow-up to at least 3 months from recovery and performed CMR and interpreted the data at our institute to lessen bias and observer variability.
During the time of COVID-19, there were no specific parameters that would accurately rule out other factors for hospitalisation or other markers, such as troponin and natriuretic peptide, which could account for possible confounders and lack of the specificity of myocardial injury.
In addition, the study was a one-point-in-time CMR examination. Thus, follow-up on the status of cardiac involvement and prognosis was not included in the study. As there was no established protocol during the study period, the physician’s decision was final. Determining the association of clinical and CMR parameters with the risk of developing myocarditis is beyond the scope of this study.
The inadequacy of the sample size was also a limitation. The sample size was limited primarily because of the diagnostic cost of performing CMR. Also, its role in COVID-19 patients is currently unclear; thus, there were few physicians who prescribed/recommended this diagnostic imaging for this subset of patients. In addition, appropriate documentation of the timing of the onset of cardiac symptoms to the time of CMR examination will provide more accurate results regarding the development of myocarditis in short- or long-term recovered COVID-19 patients. Nevertheless, the findings of the study still present the imperative need of continuous monitoring of recovered COVID-19 patients who develop persistent symptoms that may be due to cardiac involvement. Further studies are recommended in a larger population to identify clinical outcomes. It is essential to conduct longitudinal follow-up using the same diagnostic imaging techniques to fully understand the progression or resolution of myocarditis in patients who have recovered from COVID-19.
Conclusion
Myocarditis after COVID-19 can be a lasting consequence, and CMR may serve as a sensitive imaging tool to investigate any suspected cardiac injury after treatment of the infection. The major CMR findings were myocardial oedema and myocardial fibrosis.
There was no difference between the right and left ventricular parameters, although some patients may have significant impaired right ventricular EF function. Patients who have recovered from COVID-19 may warrant closer monitoring, especially those who have persistent cardiac symptoms of chest pain.
Clinical Perspective
- Cardiac magnetic resonance (CMR) results highlight the severe and significant cardiac injury following recovery from COVID-19.
- This study highlights that patients recovered from COVID-19 demonstrate myocardial oedema and late gadolinium enhancement consistent with the presence of on-going active myocardial injury.
- It is crucial to identify significant changes by CMR imaging that may be less obvious on routine testing with echocardiography.
- Clinicians may consider CMR in patients reporting on-going cardiac symptoms after recovery to assess myocardial health and optimise treatment.
- This may aid in determining cardiac residual damage, providing an effective means for treating on-going cardiovascular sequelae associated with COVID-19.