Sudden cardiac death (SCD) is a leading cause of death worldwide and is commonly precipitated by acute ventricular tachyarrhythmia episodes induced by acute MI.1 It has been observed that ventricular arrhythmia (VA) is the most common cause of SCD in patients with acute MI, with a 10-fold increase over the first 30 days.1 Early ventricular tachycardia (VT) and VF are linked to greater in-hospital mortality.
Certain ECG abnormalities during acute MI are related to fatal VA, and numerous attempts have been made to anticipate their occurrence, because early recognition of people at a high risk and prompt implementation of aggressive treatment strategies can save lives.2,3
One ST-segment abnormality, the lambda-like ST-elevation pattern, has gained attention in recent years due to its association with VA in acute MI patients.4 This ECG pattern was first reported in 2004 and discussed by Gussak et al.5 As it is characterised by QRS complexes with slurry upsloping and downsloping limbs resembling the Greek letter λ (lambda), it was named the lambda-like ST elevation, which is also known as shark fin ST-elevation.5,6 Several retrospective studies have indicated a strong association between this ECG pattern and fatal VA during acute MI.7–9
We describe a case of fatal VA in a patient with acute MI with a lambdalike ST-elevation pattern.
Case Presentation
A 49-year-old man with poorly managed type 2 diabetes and hypertension arrived at our emergency department presenting with 3 days of intermittent, worsening angina. He presented within an hour of the most severe angina.
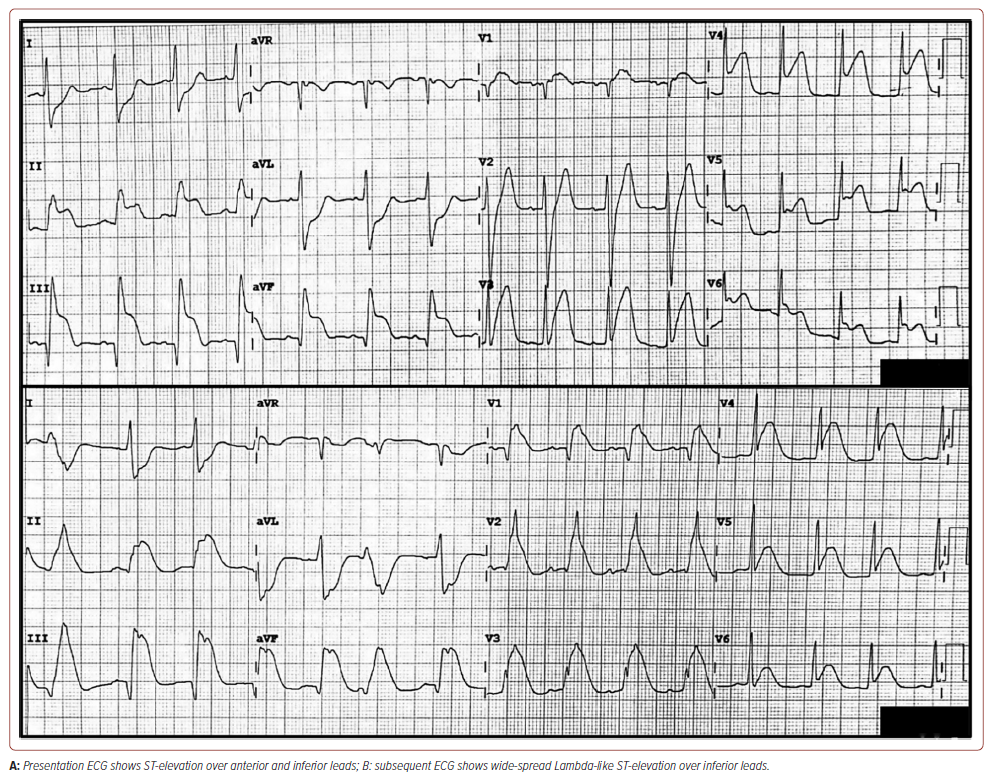
The clinical examination revealed a blood pressure of 119/79 mmHg, a pulse rate of 94 BPM, a respiratory rate of 24 breaths/minute, oxygen saturation (SpO2 ) of 97% under nasal prong 3 l/min and a pain score of 8/10. The extremities were warm and without oedema; however, the jugular vein was distended. Lung examination revealed bilateral basal crepitation; cardiac examination revealed dual rhythm with no murmur. An initial ECG showed PR elevation of lead aVR, ST elevation over leads V3 to V6, II, III and aVF, and hyperacute T wave over V2 to V3 with reciprocal ST depression in the lead I and aVL. It then evolved into widespread lambdalike ST-elevation of V1 through V6, II, III and aVF (Figure 1). High-sensitive troponin T was elevated at 582 ng/l (<14 ng/l). The other blood test result was unremarkable. The bedside echocardiography showed impaired left ventricular function of 40–45%, hypokinetic anterior and inferior walls with no ventricular septal rupture.
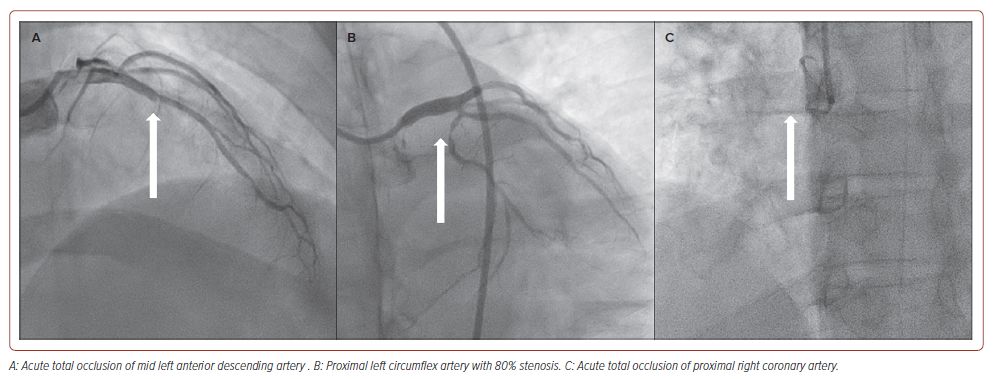
The patient was diagnosed with acute extensive ST-elevation MI, Killip II, and scheduled for primary percutaneous coronary intervention (PCI). During preparation, he developed recurrent episodes of VT, followed by VF, which required 20 minutes of cardiopulmonary resuscitation (CPR), five cycles of defibrillation, IV norepinephrine 5 mg and IV amiodarone 450 mg before the return of spontaneous circulation. He was intubated and haemodynamically supported by IV norepinephrine and dobutamine before undergoing coronary angiography. The coronary angiography revealed acute total occlusion (ATO) of the mid-left anterior descending artery (LAD), complete acute occlusion of the proximal right coronary artery (RCA) and proximal left circumflex artery with 80% stenosis (Figure 2).
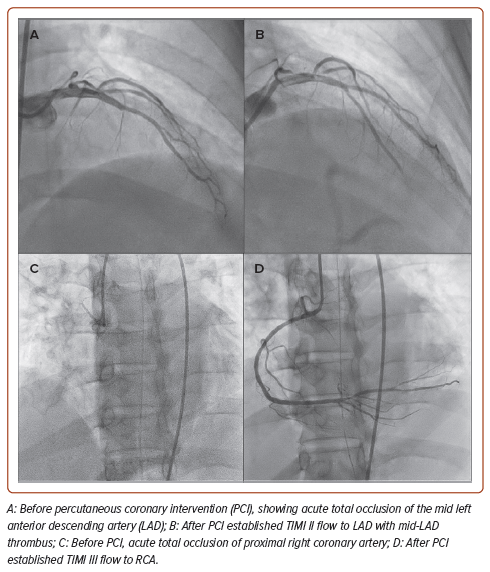
We performed PCI for the RCA and the LAD ATO. Due to the lack of other mechanical circulatory devices available at our centre, an intra-arterial blood pump was inserted. The RCA was engaged with a 6 Fr Judkins right guiding catheter via the right femoral artery, and a hydrophilic coronary guidewire was easily advanced to the distal RCA. Due to an unusually high thrombus burden, we performed thrombus aspiration using an aspiration catheter and successfully aspirated multiple white thrombi. TIMI-II flow was restored after thrombus aspiration. During preparations to stent the proximal RCA, the patient developed VF, necessitating an additional 15 minutes of CPR, 3 mg of IV epinephrine and two defibrillations before return of spontaneous circulation. The proximal RCA was then stented with a 2.75 mm × 18 mm everolimus-eluting stent. After post-dilatation with a stent balloon, TIMI-III flow was restored in the RCA prior to LAD PCI. The duration of RCA PCI was 12 minutes.
We then engaged the left coronary artery with a 6 Fr extra backup Judkins left guiding catheter and wired down the LAD with a hydrophilic coronary guidewire. Again, numerous red and white thrombi were aspirated. Using a 2.5 mm × 12 mm semi-compliant balloon, we successfully re-established TIMI-II flow to the proximal LAD. As the LAD had no apparent target lesion but a thrombus-laden mid-LAD, we chose to perform percutaneous balloon angioplasty on the LAD and planned to continue with the glycoprotein IIb/IIIa infusion post-procedure.
Despite the satisfactory angiographic outcome (Figure 3), the patient experienced recurrent VT and VF episodes in between procedures. By the time the procedure had been completed, he had received 45 minutes of CPR, 10 mg IV epinephrine, 2 mg IV atropine, 2.5 g IV magnesium sulphate, 10 mEq IV sodium bicarbonate 8.4%, 100 mg IV lidocaine and the maximum doses of noradrenaline, dobutamine and dopamine. Despite successful PCI to LAD and RCA ATO, and surviving multiple episodes of resuscitation, the patient died 1 hour after the procedure.
Discussion
In recent years, because of advances in medical care, the majority of patients with acute MI have been treated with revascularisation in terms of thrombolysis or PCI, depending on their clinical status and the local resources available. Despite this, the mortality rate in acute MI patients has remained high.1
The ECG, a readily available and time-sensitive diagnostic tool, has been instrumental in assessing acute MI and predicting patient outcomes. Therefore, efforts have been made to identify distinct ECG patterns to predict the occurrence of VA, which occurs in conditions such as Brugada syndrome and short QT-interval syndrome, in an attempt to improve the standard of care for acute MI patients and reduce the mortality rate among them.6
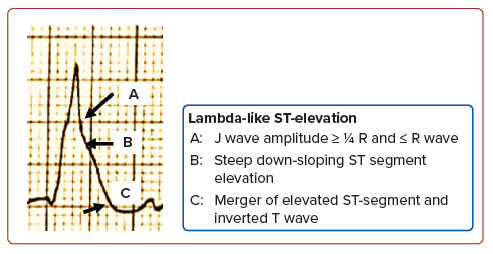
The lambda-like ST-elevation has been linked to deadly VA and cardiogenic shock with a high in-hospital mortality rate.2–5,7–9 Wang et al. defined this as an elevated J wave (amplitude ≥1/4 R wave and ≥R wave) followed by a steep, down-sloping elevated ST-segment that merges with the inverted T wave, forming a lambda shape with the range of the included angle between the isoelectric line and tangent line (connecting the J point and the convex point of the J–ST–T complex) of 0–90° (Figure 4).4 This facilitates the recognition of this ECG rhythm, as identification of the J-point in ST-elevation can be challenging at times.8
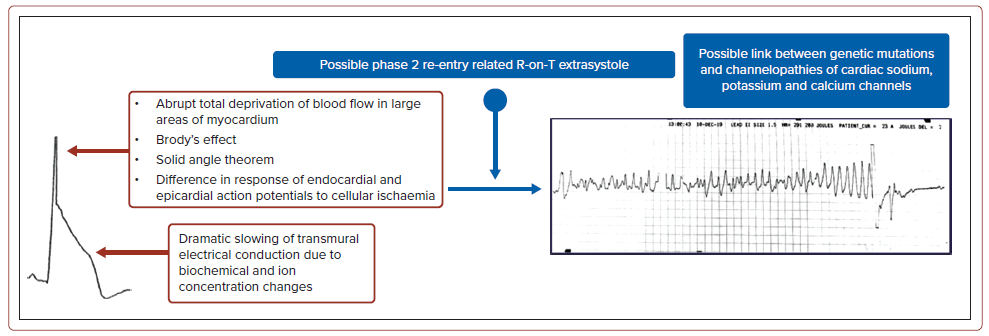
The electrophysiological explanation for ST-elevation in lambda-like rhythms remains hypothetical. The rise in R wave amplitude with elevated J wave and the steep, down-sloping ST-elevation can be divided into two portions. First, the tall R wave has been observed in individuals with abrupt, complete blood flow deprivation to extensive areas of the myocardium. It may also have single or multiple explanations, such as: the expansion of left ventricular cavity volume in acute MI – Brody’s hypothesis; the solid angle theorem, in which the potential voltage difference between ischaemic and normal myocardial tissue is explained by tissue resistance affecting the myocardial conductivity factor; or the difference in response of endocardial and epicardial action potentials to cellular ischaemia, due to differences in transient outward current (Ito), IK, adenosine triphosphate and early INa activity and availability.5,8,9 Next, during acute ischaemia, the inward sodium and calcium currents decrease, resulting in an increased augmentation in the Ito, which creates transmural or spatial heterogeneity of voltage gradients and results in J waves and ST-segment elevations on the ECG.7
During an acute MI, biochemical and ion concentration imbalances may dramatically slow transmural electrical conduction, resulting in a steeply down-sloping ST-segment elevation. This builds up the voltage gradient in the early stages of acute myocardial ischaemia, which can lead to the R-on-T phenomenon and, eventually, VF.10 These explain the lambda-like ST-elevation on the ECG. In addition, reports have suggested a link between genetic abnormalities and channelopathies with lambda-like STelevation resulting from early aberrant ventricular repolarisation (Figure 5).4 Therefore, we hypothesised that lambda-like ST-elevation may serve as a visual predictor of fatal VA, cardiogenic shock and sudden cardiac death in patients with acute MI.
While lambda-like ST-elevation holds promise as a visual predictor of adverse outcomes in acute MI patients, it is important to acknowledge its limitations. One is that lambda-like ST-elevation is not always present in patients who experience these adverse events. Additionally, the presence of lambda-like ST-elevation may not be specific to acute MI; it can also be seen in other conditions such as myocarditis or pericarditis.
Conclusion
Overall, lambda-like ST-elevation is a potentially valuable diagnostic and prognostic tool in acute MI patients. Further research is needed to elicit the underlying mechanisms and refine its clinical utility. Our case demonstrated that a Lambda-like ST-elevation ECG in acute MI may be a risk predictor of fatal VA, cardiogenic shock and sudden cardiac death, and alert clinicians to adopt early aggressive treatment strategies to reduce morbidity and mortality.
Clinical Perspective
- Lambda-like ST-elevation on the ECG in acute MI may be a predictor of the risk of fatal ventricular arrhythmia, cardiogenic shock and sudden cardiac death.
- This finding highlights the importance of recognising lambda-like ST-elevation as a warning sign of potentially life-threatening complications in patients with acute MI.
- Early identification of lambda-like ST-elevation in acute MI patients is crucial for prompt intervention and improved patient outcomes.
- Recognising this ECG pattern should alert clinicians to adopt aggressive treatment strategies, including early reperfusion therapy, antiarrhythmic medications and haemodynamic support to reduce the risk of adverse events.