Heart failure (HF) is a clinical syndrome characterised by the inability of the heart to perform circulatory function efficiently due to structural and/or functional abnormalities.1,2 It continues to be an important global health issue and is estimated to affect nearly 23 million people worldwide.1,2 With an epidemic of coronary artery disease, diabetes and other lifestyle diseases, it is estimated that the number of HF patients globally will increase by 25% by the year 2030.3
Iron deficiency (ID) is an extremely common comorbidity in patients with heart failure. One-third of HF patients are anaemic and almost 50% have ID.4 ID is associated with more HF symptoms, worse New York Heart Association (NYHA) functional status, greater risk of HF hospitalisation and reduced survival.2 It is also associated with reduced exercise capacity and physical wellbeing, and reduced quality of life.
ID is also a common comorbidity in patients with HF with reduced ejection fraction (EF), and has been associated with increased mortality and a poorer quality of life, regardless of whether there is concomitant anaemia.5 As to whether these are the mediators of poor outcome or are just the bad prognostic markers, the debate is far from over.6
Iron studies are already a part of the routine check-up of patients, and it is now recommended that all patients with HF are regularly screened for anaemia and ID with full blood count, serum ferritin concentration and transferrin saturation.7
Most studies on the prevalence of ID associated with HF are from Europe and North America. Currently, there are not enough data from Nepal or the south Asian region to permit an estimation of the prevalence of ID associated with HF. This study intended to assess the prevalence of ID in HF in this region, and may help formulate future guidelines in Nepal and south Asia for routine ID assessment in HF patients.
Objectives
The primary objective of the present study was to estimate the prevalence and spectrum of ID in HF patients with or without anaemia.
The secondary objective of the present study was to find the associations of HF with ID.
Materials and Methods
This study was a single-centre observational study, conducted at Shahid Gangalal National Heart Centre, Kathmandu, Nepal.
Following ethical clearance from the Institutional Review Board, male or female patients aged >18 years and clinically diagnosed with HF, who gave consent for the study, were included.
The diagnosis of HF was established on the basis of validated clinical criteria from the 2021 European Society of Cardiology guidelines for the diagnosis and treatment of acute and chronic HF.
All participants underwent a thorough history (including dietary history) and clinical evaluation, blood sampling and comprehensive transthoracic echocardiography using standardised equipment. Patients were characterised as having normal EF (EF ≥50%), reduced EF (EF ≤40%) or mildly reduced EF (EF 41–49%). Apart from the routine haemogram, these patients were assessed for their iron status by measuring the complete iron profile, including serum iron, serum ferritin, total iron binding capacity and transferrin saturation.
Anaemia was defined as haemoglobin (Hb) <13 g/dl for men and <12 g/dl for women, based on the WHO definition.
ID in HF was defined as either having depleted iron stores – low ferritin (<100 µg/dl) and low transferrin saturation (≤20%) – or having functional ID in the form of normal iron stores (ferritin 100–300 µg/dl) or low transferrin saturation (≤20%).
This was a cross-sectional study. Using a prevalence of ID in HF of 50%, as shown by previous studies, the z-value of 1.96 for a 95% CI and a margin of error of 5%, the calculated sample size was 383. Convenience sampling was used in this study. The duration of the study was 6 months. Inclusion criteria were adults aged >18 years clinically diagnosed as having heart failure, and those willing to give consent. Exclusion criteria were having comorbid noncardiac conditions causing ID (e.g. haemorrhoids, malignancy etc.), primary valvular heart disease, taking anticoagulants, end-stage renal disease, peripartum status, congenital heart disease and patients not willing to give consent. The primary outcome of this study was the prevalence and pattern of ID in HF. The secondary outcome was associations of ID with HF.
Statistical Analysis
All data were entered into an electronic spreadsheet and analysed using SPSS version 20. Categorical data were expressed as the frequency and percentage, and continuous data as the mean. Results were analysed using the χ2 test to compare categorical variables. A logistic regression model was used to calculate the odds ratio with a predetermined level of significance of 0.05 and 95% CI.
Results
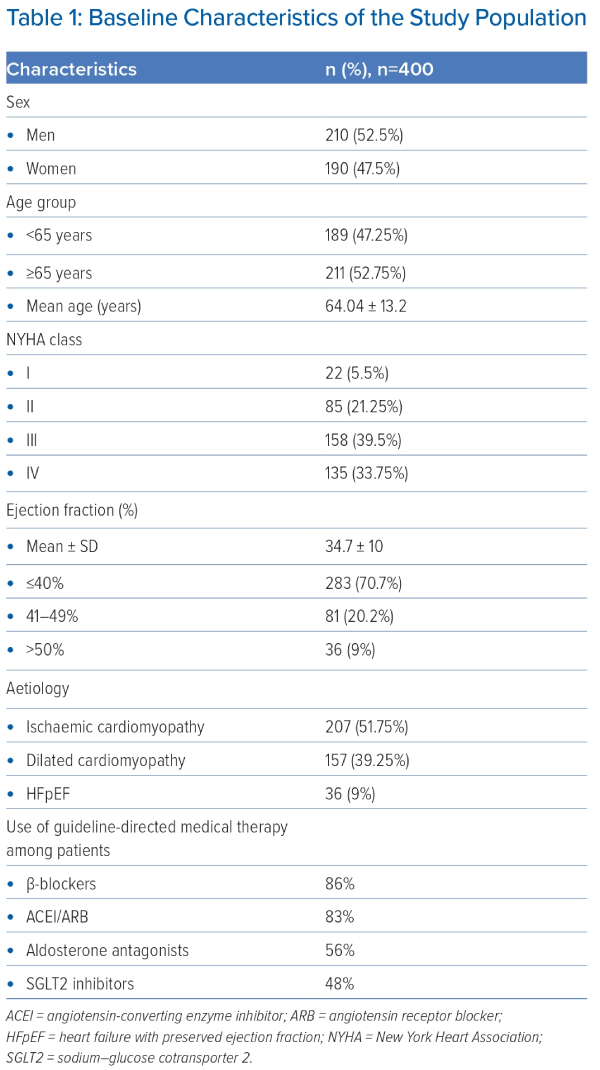
During the study period, 400 patients either presenting to the outpatient department or admitted to the hospital were included in the study. The baseline characteristics of the patients are shown in Table 1. There were 210 (52.5%) men and 190 (47.5%) women. The mean age of the patients included was 64.04 ± 13 years and mean EF 34.69 ± 10%.
The majority of the patients (73.25%) presented with NYHA class III or IV. Ischaemic cardiomyopathy was present in 47.2% (189) of the patents, followed by dilated cardiomyopathy in 43.8% (175). In total, 36 (9%) of the patients had HF with preserved EF. The majority (70.7%) of the patients had reduced left ventricular EF, whereas mildly reduced EF was present in 20.2% of the patients.
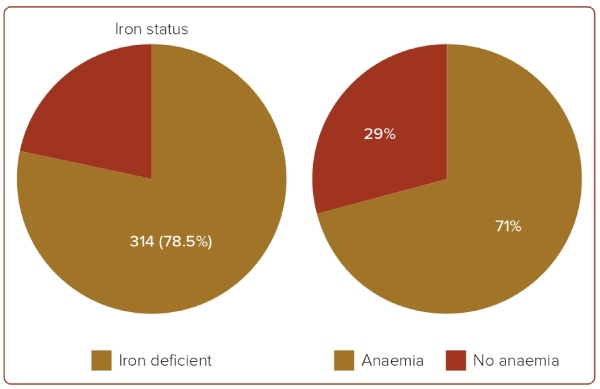
Anaemia was present in 244 (61%) of the patients, of whom 112 (46%) were men and 132 (54%) were women. Compared with those without anaemia, patients with anaemia had a much higher prevalence of ID.
Severe anaemia (Hb <8 g/dl) was present in 15 (6.1%), moderate anaemia (Hb 8–10 g/dl) in 60 (24.5%) and mild anaemia (Hb 10–12/13) in 169 (70.9%) patients (Supplementary Figure 1).
ID was present in 314 (78.5%) of the total included patients, of whom 161 (51.2%) were women and 153 (48.7%) were men. Concurrent anaemia was present in 222 (71%) of the patients with ID (Figure 1).
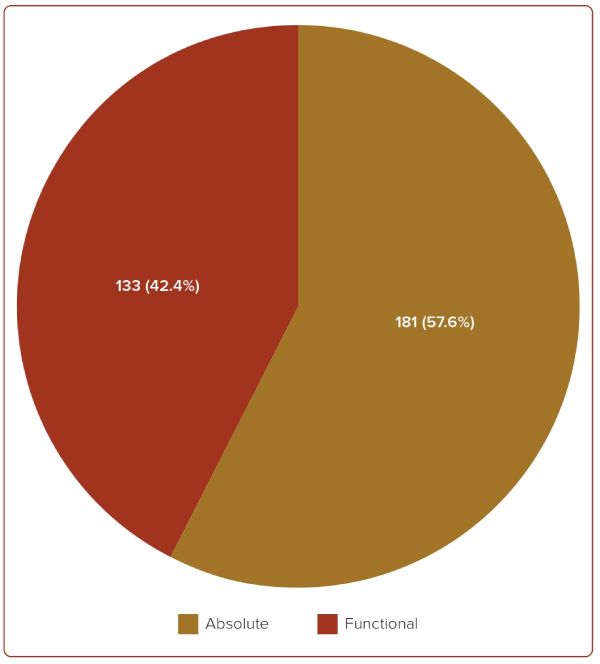
Absolute ID was present in 181 (57.6%) patients, and functional ID was present in 133 (42.4%) patients, as shown in Figure 2. Out of the 181 patients with absolute ID, concurrent anaemia was present in 76% (137) of the patients, and out of the 133 patients with functional ID, concurrent anaemia was present in 85% (64) of the patients (Supplementary Figures 2 and 3). Overall, ID without anaemia was present in almost one-third (29%) of the patients.
Patients with ID with or without anaemia were stratified (Supplementary Table 1).
ID with anaemia was present in 222 (71%) of the patients, of whom absolute ID with anaemia was present in 137 (76%) and functional ID with anaemia was present in 85 (64%) patients.
Of the 86 patients who had normal iron levels, 22 (5.5%) had anaemia.
ID without anaemia was present in 92 (29%) patients, signifying the importance of screening for iron levels even if the patient does not have anaemia.
Patients with ID were further classified according to their sex, NYHA functional class, age and LV function (Table 2).
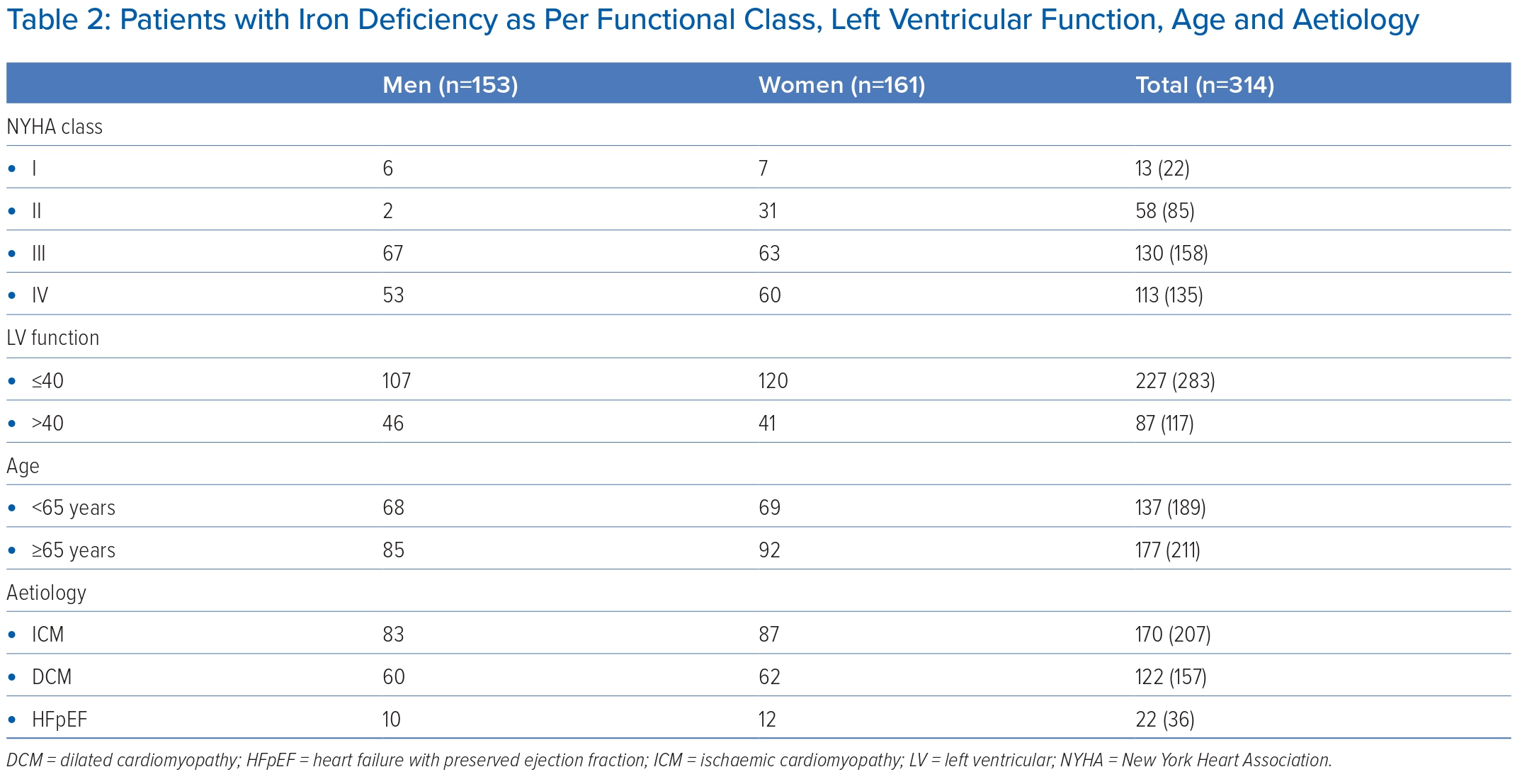
ID was present in 113 (83.7%) of the patients with NYHA IV, 130 (82.27%) with NYHA III, 58 (68.23%) with NYHA II and 13 (59%) with NYHA I. Similarly, ID was present in 227 (80%) patients with an EF <40%, whereas 87 (74.3%) patients with EF >40% were iron deficient.
ID was present in 177 (83.8%) of the patients who were aged >65 years, and 137 (72.4%) of the patients aged <65 years. ID was present in 170 (82.1%) patients who had ischaemic cardiomyopathy and 122 (77.7%) patients with dilated cardiomyopathy, whereas 22 (61.1%) patients with HF with preserved EF had ID.
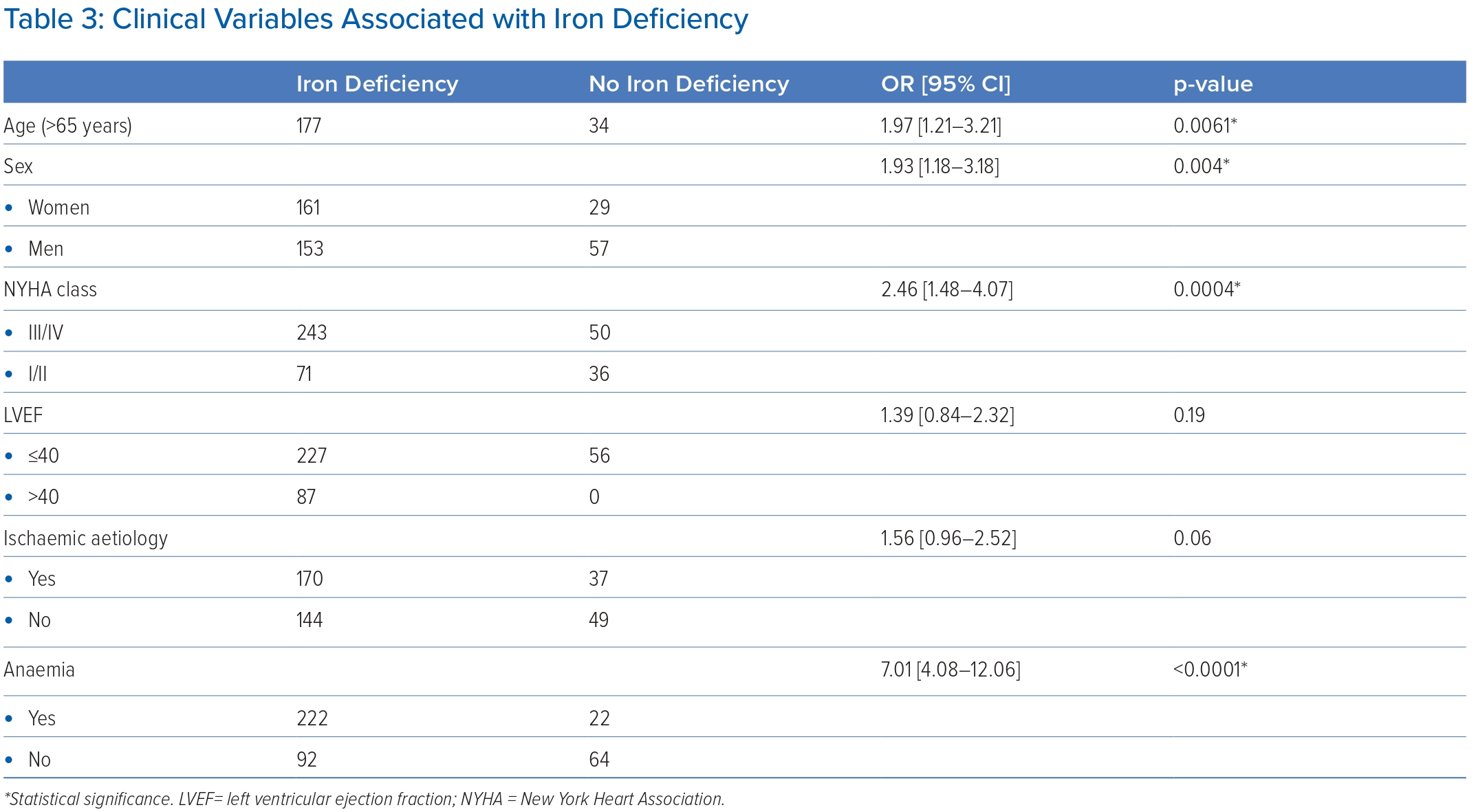
The clinical variables associated with ID – OR with 95% CI – are shown in Table 3. ID had a significant and independent association with age >65 years (OR 1.97; 95% CI [1.21–3.21]; p=0.0061), female sex (OR 1.93; 95% CI [1.18–3.18]; p=0.004), patients presenting with NYHA class III or IV (OR 2.46; 95% CI [1.48–4.07]; p=0.0004) and presence of anaemia (OR 7.01; 95% CI [4.08–12.06]; p<0.0001).
Patients with LV function of ≤40% (OR 1.39; 95% CI [0.84–2.32]; p=0.19) or ischaemic aetiology (OR 1.56; 95% CI [0.96–2.52]; p=0.06) were more likely to have ID, but these were not statistically significant.
Discussion
Our data on the iron status of 400 patients with HF in a tertiary cardiac centre of Nepal showed a notably high prevalence of ID.
ID is one of the most common nutritional deficiencies worldwide, affecting approximately one-third of the general population.8 The past decades have seen tremendous research effort into ID in patients with chronic diseases with underlying inflammatory activation, and these efforts have finally yielded the understanding that patients with HF are at increased risk of developing ID.9
Various studies performed in developed nations have shown an estimated prevalence of ID at approximately 50% independent of the presence of anaemia.9,10 ID may impair functional capacity, precipitate decompensation, promote skeletal muscle dysfunction, is associated with frailty, and is independently associated with reduced exercise capacity, recurrent HF hospitalisations, and high cardiovascular and all-cause mortality irrespective of anaemia.11 A meta-analysis of 153,180 patients conducted in patients with HF showed that the crude mortality risk of anaemia had an OR of 1.96.12
Our study showed that the prevalence of ID stands at 78.5%, which is much higher than that of developed nations. Studies conducted in south Asia also showed similar findings.13,14 A study conducted in a multi-ethnic Asian population also suggested that HF patients of south Asian ethnicity had higher rates of ID.15
Studies showed that approximately 35–78% of HF patients suffer from malnutrition, which has also been independently associated with adverse outcomes.16,17 In a large international cohort of 2,357 patients with worsening HF, decreased estimated protein intake was used as a surrogate to estimate dietary iron intake. It was found that patients with lower estimated protein intake have a higher prevalence of ID, indicating poor nutritional status as a potential cause of ID in HF.18
Another study assessing the dietary intake of iron in HF patients using a 4-day food diary found that iron intake was lower than the recommended intake in 46% of patients, with significantly lower dietary intake in patients with NYHA class III–IV as compared with class II.19 In a study of 226 HF with reduced ejection fraction patients from India, it was found that HF patients that consume a vegetarian diet were 2.5-fold more likely to have ID.20
Although our study showed no such relationship with a vegetarian diet, it can be said that lower dietary intake of iron and a poorer nutritional status is associated with a higher prevalence of ID and more adverse outcomes of HF, which can be potentially explained by the dietary habits of the south Asian population.15,16
Multiple possible causes of ID in HF have been described, which include reduced oral intake, persistent systemic inflammation causing increased cytokines eventually causing reduced erythropoietin production and response, hepcidin-induced decreased gut absorption and trapping of iron in iron stores in the macrophages and hepatocytes causing decreased availability.21
The most prominent role of iron is in oxygen homeostasis, including oxygen transport and storage, oxidative metabolism in skeletal and cardiac muscle, mitochondrial function and maintenance of cellular energy and metabolism.22,23
Cardiac myocytes as tissue with high energy demand are especially sensitive to limited iron usage and decreased iron supplies, which leads to impairment of cellular myocyte function that has impacts on HF pathophysiology.24
Routine assessment of iron status is now recommended in all HF patients in internationally accepted guidelines. Treatment of ID with intravenous iron supplementation with ferric carboxymaltose in symptomatic patients with reduced left ventricular EF and ID is a class IIa recommendation in the European Society of Cardiology guidelines for HF.11 Several studies have shown that iron supplementation improves overall quality of life with fewer symptoms, improved exercise capacity and fewer heart failure hospitalisations.25–27 Meta-analysis of randomised controlled trials showed a reduction in cardiovascular death or HF hospitalisation in iron-treated patients that remained irrespective of the presence of anaemia.28–30 Such trials are lacking in the south Asian population, and our study identifies the need for future large-scale multinational observational and randomised interventional studies in Nepalese and south Asian populations.
Limitations
This study is a single-centre study conducted at a tertiary care centre in Nepal, so it would be difficult to generalise the findings necessitating larger multicentre studies. Also, the limitations of the observational nature of our study need to be acknowledged.
Conclusion
Our study showed a very high prevalence of ID in HF patients, which was significantly associated with older age, female sex and higher NYHA class. It also showed that a significant number of patients who had normal haemoglobin levels had ID, which is often overlooked. Therefore, iron profiling should be a part of the routine assessment of patients with HF. 
Click here to view Supplementary Material
Clinical Perspective
- The prevalence of iron deficiency in heart failure patients in Nepal and south Asia is even greater than in developed nations.
- The associations with heart failure, as described in the study, will help clinicians in assessment and prognosis.
- Iron profiling, although recommended in guidelines, is still not a part of the routine assessment of patients with heart failure.
- The recommended IV formulation of iron is still not available in Nepal and with data showing the high prevalence of iron deficiency efforts need to be made to make it available to patients.