Right ventricular (RV) involvement resulting from acute inferior wall MI plays a crucial role in predicting adverse cardiovascular events, including arrhythmias, cardiogenic shock, heart failure and mortality.1 However, conventional echocardiographic measures of RV function often exhibit abnormalities only after overt dysfunction has manifested due to the intricate anatomy and asymmetric shape of the RV.2 To address these limitations, longitudinal strain has emerged as a promising approach, capable of detecting subtle RV dysfunction.3 The aim of this study was to evaluate RV function using free wall longitudinal strain (fwLS) in patients with inferior wall MI and explore its correlation with major adverse cardiovascular events (MACE) and angiographic characteristics.
Methods
This was a prospective observational study conducted in the Department of Cardiology, Government TD Medical College (Alappuzha, India) in accordance with the Declaration of Helsinki. The study was approved by the Ethics Committee of Government TD Medical College, Alappuzha (ECR/122/Inst/KL/2013/RR-2016/EC 63).
This prospective study enrolled consecutive patients aged 18–80 years who presented with symptoms and signs of inferior wall ST-elevation MI (IWSTEMI), confirmed via ECG, and underwent primary percutaneous coronary intervention (PCI) within 12 hours of symptom onset. Patients were excluded from the study if they had bundle branch blocks, chronic kidney disease, established lung disease, previous PCI, rheumatic heart disease, improper image acquisition, and poorly visualised endocardial borders (Figure 1A).
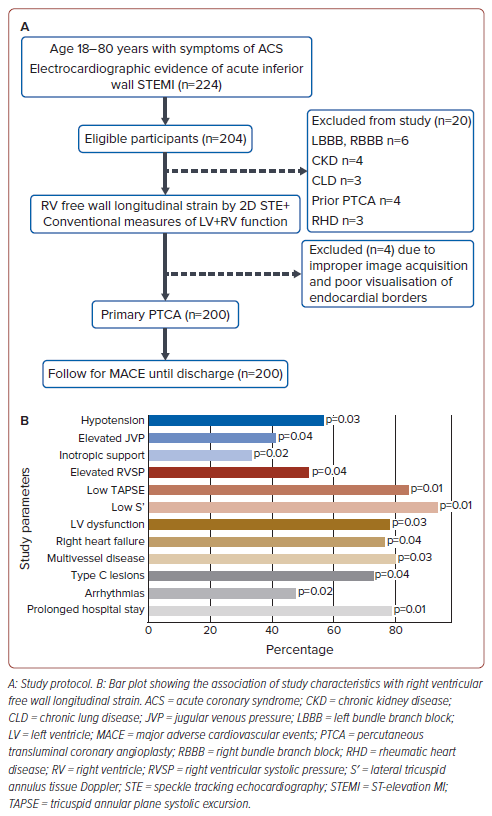
The primary objective of the study was to evaluate the prognostic significance of RVfwLS in predicting individual components of MACE among patients who have undergone successful primary angioplasty for their first IWSTEMI. The MACE analysed in our study were:
- Arrhythmias, including atrioventricular block (Mobitz type 1 and 2), complete heart block, atrioventricular and junctional tachyarrhythmias.
- Right heart failure, defined as elevated jugular venous pressure (JVP) with prominent V waves and Y descent, an auscultatory RVS3 and 2D-transthoracic echocardiographic evidence of tricuspid annular plane systolic excursion (TAPSE) <17 mm, S′ <9 cm/s with a non-collapsible inferior vena cava diameter >21 mm.
- Re-infarction – ST-segment elevation MI (STEMI), defined as recurrent clinical signs and symptoms of ischaemia with concomitant ECG changes and serum biomarker evidence of myocardial necrosis occurring within 28 days of an incident or recurrent MI.4,5
- Target lesion revascularisation (TLR), defined as a repeat PCI of the target lesion (treated segment including the 5 mm margin proximal and distal to the stent/scaffold) or bypass surgery of the target vessel performed for restenosis or other complication of the target lesion.4,5
- Recurrent angina, defined as recurrence of chest pain subsequent to PCI during the course of the admission period.6,7
- Duration of hospital stay, with a ‘prolonged hospital stay’ defined as a hospital stay of >5 days.
- Death (cardiovascular and non-cardiovascular).
The secondary objective of the study was to investigate the association between RVfwLS and angiographic characteristics of the study population. In addition, we sought to evaluate whether patients with normal conventional measures of RV function but reduced RVfwLS differ in MACE and angiographic characteristics from those with normal conventional measures of RV function and normal RVfwLS.
IWSTEMI, RV infarction and posterior wall infarction were diagnosed on the basis of ECG criteria are as follows:
- IWSTEMI: ST elevation >0.1 mV in leads II, III, aVF and reciprocal ST depression in leads I and aVL.
- RV infarction: ST elevation of 0.1 mV in any of the three leads V4R–V6R, ST elevation in V1 with ST depression in V2 (discordant relationship).8
- Posterior wall STEMI: ST elevation of 0.1 mV in leads V7–V9 and an R/S ratio >1 in V1 and V2, with >0.1 mV ST depression and terminal T wave positivity.
RVfwLS was assessed at admission using 2D speckle tracking echocardiography (STE) using a GE VIVID E9 machine with ECHOPAC software. The region of interest was manually traced in the RV-focused view, encompassing the lateral tricuspid leaflet insertion point, RV apex and medial tricuspid leaflet insertion point. RVfwLS was then calculated as the average of three myocardial segments of the free wall, excluding the interventricular septum (Supplementary Figure 1). The estimated RV systolic pressure was derived from the modified Bernoulli equation, using the tricuspid regurgitation jet and right atrial pressure from the collapsibility of the inferior vena cava during phasic changes with respiration. Moreover, other conventional measures of left ventricular (LV) and RV function were analysed following the 2015 American Society of Echocardiography and European Association of Cardiovascular Imaging guidelines and recommendations for chamber quantification by echocardiography in adults.6
Demographic data and baseline characteristics were collected using a structured questionnaire. The infarct-related artery was treated with primary angioplasty, whereas non-culprit artery lesions were scheduled for elective revascularisation after discharge. Patients were followed up for MACE until discharge/death.
The cut-off value to define low RVfwLS was established based on a pilot study involving 75 healthy subjects with no cardiovascular comorbidities who had a mean (± SD) BMI of 23.44 ± 3.54 kg/m2, blood pressure 118.64 ± 12/80.96 ± 6.18 mmHg and heart rate 72.10 ± 10.89 BPM. In that pilot study, the mean RVfwLS was determined to be −23.2 ± 2.1% (Supplementary Table 1). RVfwLS less than −20% was considered low.
Statistical Analysis
All collected data were systematically coded and input into Microsoft Excel, followed by comprehensive analysis using SPSS Statistics V22. Quantitative variables were summarised using the mean ± SD or median with interquartile range (IQR), depending on the normality of distribution. Categorical variables are described using frequencies and percentages. Associations between categorical variables were assessed using the Chi-squared test or Fisher’s exact test, as appropriate. Logistic regression analysis was used to investigate the predictive value of RVfwLS for individual components of MACE. Parameters significant in the logistic regression were analysed via ROC to identify the optimal RVfwLS cut-off value for predicting MACE, aiming to maximise sensitivity and specificity. Significance was set at p<0.05(two-tailed).
Results
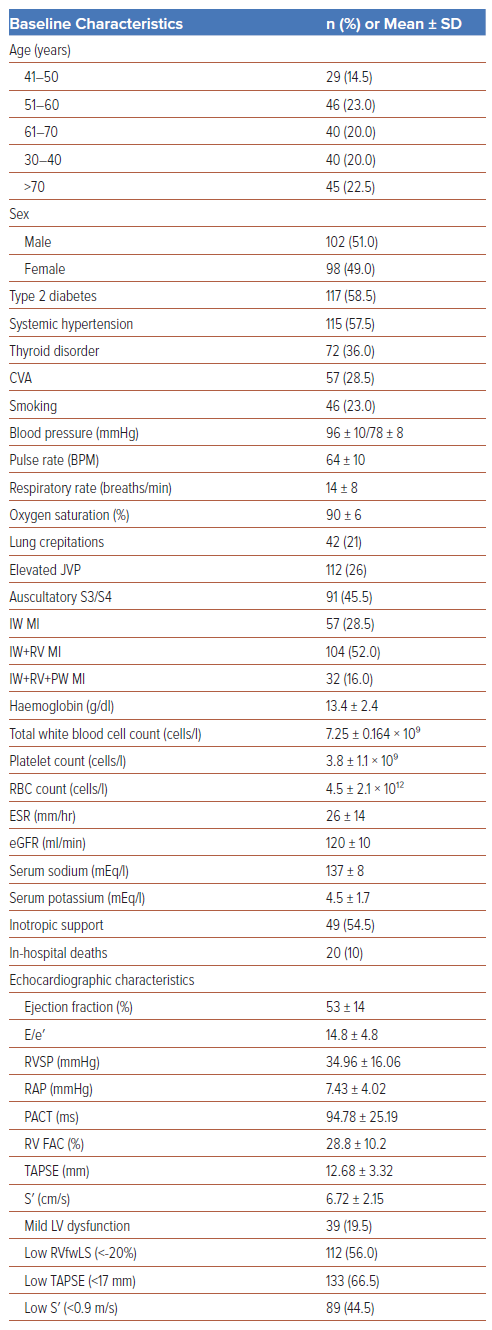
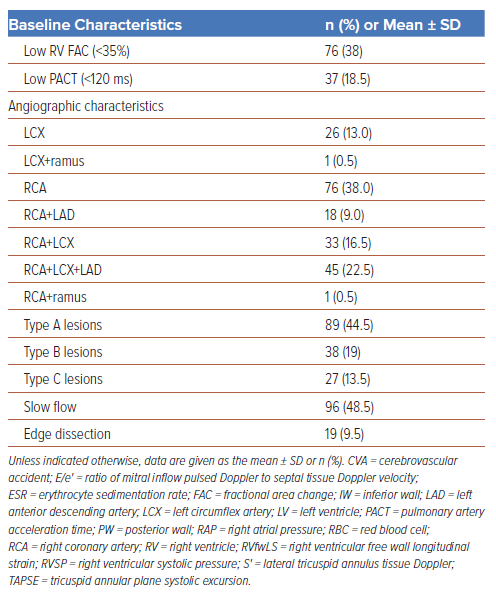
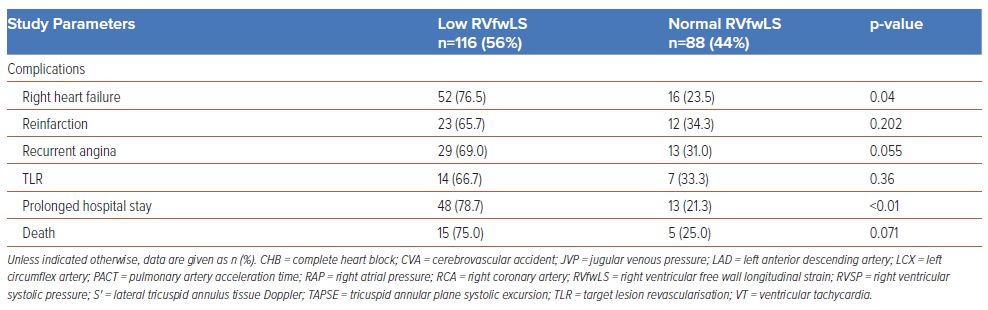
In all, 200 patients were enrolled in the study, of whom 102 (51%) were male and 98 (49%) were female. The baseline characteristics of the study population are summarised in Table 1. The mean RVfwLS was −16.50 ± 3.84%, and 112 (56%) patients had low (<−20%) RVfwLS. Of the patients with low RVfwLS, 57% and 41% presented with hypotension and elevated JVP (41%), respectively, necessitating and 33% required inotropic support (Table 2 and Figure 1B). Among the entire study population, 22.5% of patients (n=45) had triple vessel disease (TVD), and Type B lesions were the predominant lesions (n=147 [73.5%]; Table 1). Patients with low TAPSE and S′ (p<0.01) and elevated estimated RV systolic pressure (p=0.036) frequently had low RVfwLS (Figure 1B).
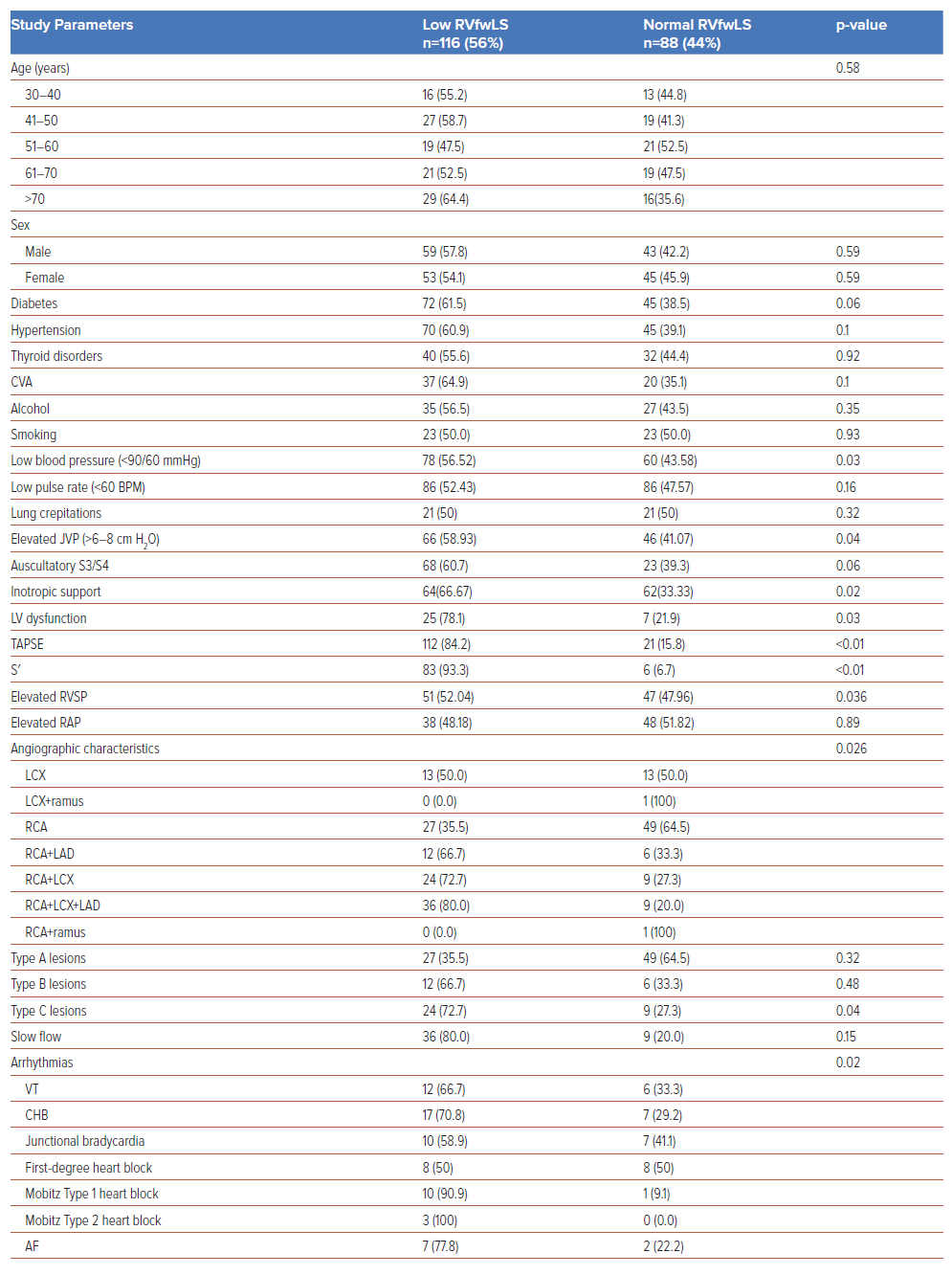
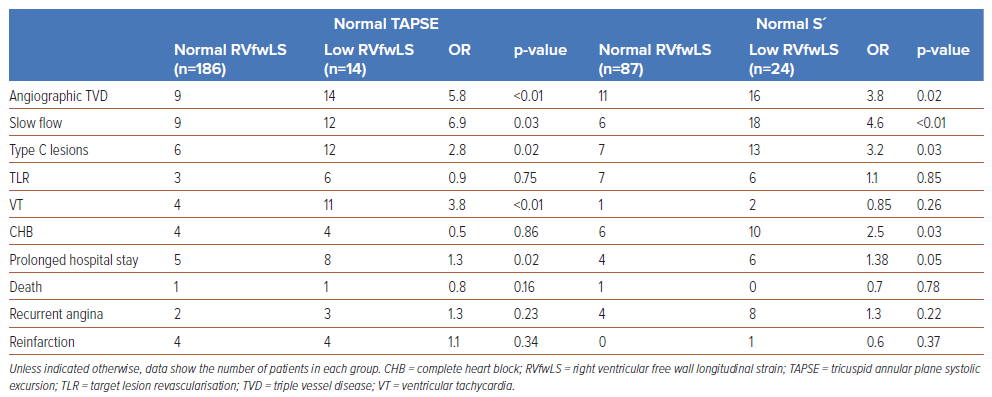
Atrioventricular blocks were the most common complication, and were seen in 33.5% of the study population. At presentation, 24 (12%) patients had complete heart block, 18 (9%) developed ventricular tachycardia and 3 (1.5%) developed Mobitz type 2 atrioventricular block. Patients with normal TAPSE but low RVfwLS had a significantly higher incidence of angiographic TVD (OR 5.8; p<0.01), slow flow (OR 6.9; p=0.03), Type C lesions (OR 2.8; p=0.02), ventricular tachycardia (OR 3.8; p<0.01), and a prolonged hospital stay (OR 1.3; p=0.02) compared with patients with normal TAPSE and normal RVfwLS. Similarly, comparisons between patients with normal S′ and low RVfwLS with normal S′ and normal RVfwLS revealed significant differences in angiographic TVD (OR 3.8; p=0.02), slow flow (OR 4.6; p<0.01), Type C lesions (OR 3.2; p=0.03), complete heart block (OR 2.5; p=0.01) and prolonged hospital stay (OR 1.38; p=0.05; Table3).
Recurrent angina, re-infarction, and TLR were observed in 42 (21%), 32 (17.5%) and 21 (14%) patients, respectively. A prolonged hospital stay was reported in 30.5% of the study population, and 10% of the study population died due to complications. RVfwLS was significantly associated with TVD (p=0.026), Type C lesions (p=0.04), arrhythmias (p=0.02), right heart failure (p=0.04) and a prolonged hospital stay (p<0.01; Figure 1B; Table 2). However, RVfwLS was not significantly associated with cardiovascular comorbidities, re-infarction, TLR, recurrent angina or mortality.
Logistic regression analysis revealed that RVfwLS is an independent predictor of arrhythmias (OR 2.05; p=0.046) and heart failure (OR 3.64; p=0.035; Supplementary Table 2). Based on our study, the optimal RVfwLS cut-off value to predict arrhythmias was −14.8% (AUC=0.915; 95% CI [0.876–0.954]; p<0.001) with a sensitivity of 81.3% and a specificity of 86.8% (Supplementary Figure 2).
Discussion
In this analysis of 200 patients presenting with IWSTEMI, we found that adults with low RVfwLS had higher rates of arrhythmias and right heart failure than patients with a normal RVfwLS. An RVfwLS value less negative than −14.8% (e.g. −12%) indicates a worse prognosis, with a sensitivity of 81.3% and specificity of 86.8% for predicting arrhythmias in patients with IWSTEMI. Our findings highlight the importance of using free wall longitudinal strain in these patients to predict some of the components of MACE, thereby providing actionable information for clinicians to deliver high-level care for these patients.
Significantly reduced RVfwLS was noted in patients experiencing cardiogenic shock complicated by complete heart block and ventricular tachycardia (p=0.02). Notably, individuals with diminished RVfwLS frequently developed clinically relevant arrhythmias, possibly due to mechanical and electrical disparities between infarcted and viable myocardial segments. This phenomenon results in repolarisation dispersion, ultimately leading to QTc prolongation.9 RVfwLS serves as a valuable prognostic marker for identifying patients at risk of arrhythmic complications after revascularisation.
Patients undergoing uneventful primary angioplasty for the right coronary artery are typically discharged by the third day. However, patients with persistent RV dysfunction after revascularisation, necessitating inotropic support, secondary ventricular tachyarrhythmias or requiring TLR, often require a prolonged hospital stay. In the present study, 78.7% of patients who experienced a prolonged hospital stay had a low RVfwLS (p<0.01). Close monitoring of this subset after the procedure is crucial to prevent late complications rather than opting for premature discharge.
RV infarction, occurring in 30–50% of patients with IWSTEMI, is the primary cause of mortality, with a 10-year survival rate of 62% for isolated right coronary artery (RCA) disease and annual mortality risks of 2% for RCA and 3% for combined RCA and left coronary artery disease beyond the first year.10 In the present study, 76.5% of patients (n=52) with RV failure had low RVfwLS (p=0.04), and logistic regression analysis revealed that low RVfwLS independently predicted right heart failure (OR 3.64; p=0.035). In addition, 25 (78.1%) patients with low RVfwLS (p=0.006) were found to have LV dysfunction. Several mechanisms have been proposed to explain heart failure due to LV dysfunction in IWSTEMI, including multivessel disease, the reverse Bernheim effect leading to altered LV geometry and a reduction in preload, all of which can have an impact on measures of LV function.7,11 Specifically, longitudinal strain serves as a robust predictor for assessing RV recovery and late remodelling, both closely associated with subsequent cardiovascular events, such as recurrent heart failure and stent thrombosis-induced infarction.12,13 Recognising this specific subset of patients holds clinical significance, because they require prolonged reverse remodelling therapy with angiotensin-converting enzyme inhibitors, β-blockers and aldosterone antagonists for mitigating the risk of long-term adverse events like recurrent heart failure.
In a study of 200 heart failure patients with preserved TAPSE (>16 mm), Carluccio et al. found that, beyond conventional echocardiographic measures, RVfwLS of −15.3% (AUC 0.68, sensitivity 50%, specificity 80%) independently predicted outcomes like all-cause death and heart failure rehospitalisation.14 In addition, RVfwLS significantly improved risk stratification, as evidenced by a net reclassification improvement (NRI) of 0.584 at 3 years, with 68% of non-events correctly reclassified.14 In an analysis of 896 patients with coronary artery disease and significant functional tricuspid regurgitation, RVfwLS proved more effective than traditional measures like TAPSE and fractional area change (FAC) in detecting RV dysfunction (84.9% of cases) and was strongly linked to all-cause mortality during a median follow-up of 2.8 years, with a mortality rate of 49.4% (p<0.001).15 In contrast, the present study extends the utility of RVfwLS to acute clinical scenarios, emphasising its relevance in immediate clinical decision making and patient management. We established a slightly different optimal threshold for RVfwLS (−14.8%; AUC 0.915, sensitivity 81.3%, specificity 86.8%; p<0.001) for predicting arrhythmias and right heart failure.
In a study assessing RV involvement in 100 patients with acute IWSTEMI using 2D STE, RVfwLS differentiated proximal from distal RCA lesions.16 RVfwLS was significantly lower in proximal than distal lesions (−14.2 ± 4.6 versus −17.7 ± 4.2, respectively; p=0.026), with a cut-off value of ≤−20.5% showing 88% sensitivity and 33% specificity for detecting RV infarction.16 In contrast, we found that among patients with angiographically confirmed TVD (n=45 patients), a significant proportion (36.80%) exhibited impaired RVfwLS (p=0.036). In addition, in the subset of patients with Type C lesions (n=22 patients), 72.7% (29 patients) had impaired RVfwLS. Although the predictive value of RVfwLS in identifying proximal versus distal RCA occlusions has been established in previous studies16, to the best of our knowledge the association between RVfwLS and angiographic multivessel disease and their lesion characteristics has not been documented. Future research is needed to investigate whether impaired RVfwLS in patients with multivessel disease is correlated with long-term adverse outcomes.
Patients previously classified as having normal RV function based on standard echocardiographic parameters, such as TAPSE, RV FAC, and S′, may, in fact, exhibit reduced RVfwLS. In our study, 7% (n=14) of individuals with normal TAPSE and 21.6% (n=24) with normal S′ had impaired RVfwLS (p<0.001), which suggests that patients with normal TAPSE and S′ may still have subclinical RV dysfunction detectable through RVfwLS. The incidence of angiographic TVD (OR 5.8; p<0.01), slow flow (OR 6.9; p<0.03), Type C lesions (OR 2.8; p<0.02), ventricular tachycardia (OR 3.8; p<0.01) and prolonged hospital stay (OR 1.3; p<0.02) was significantly higher among patients with normal TAPSE but low RVfwLS. Similarly, those with normal S′ but abnormal RVfwLS also had a higher incidence of angiographic TVD (OR 3.8; p<0.02), slow flow (OR 4.6; p<0.01), Type C lesions (OR 3.2; p<0.03), complete heart block (OR 2.5; p<0.03) and a prolonged hospital stay (OR 1.38; p<0.05). These findings underscore the significance of measuring RVfwLS, particularly when conventional echocardiographic indicators suggest normal RV function (Table 3).
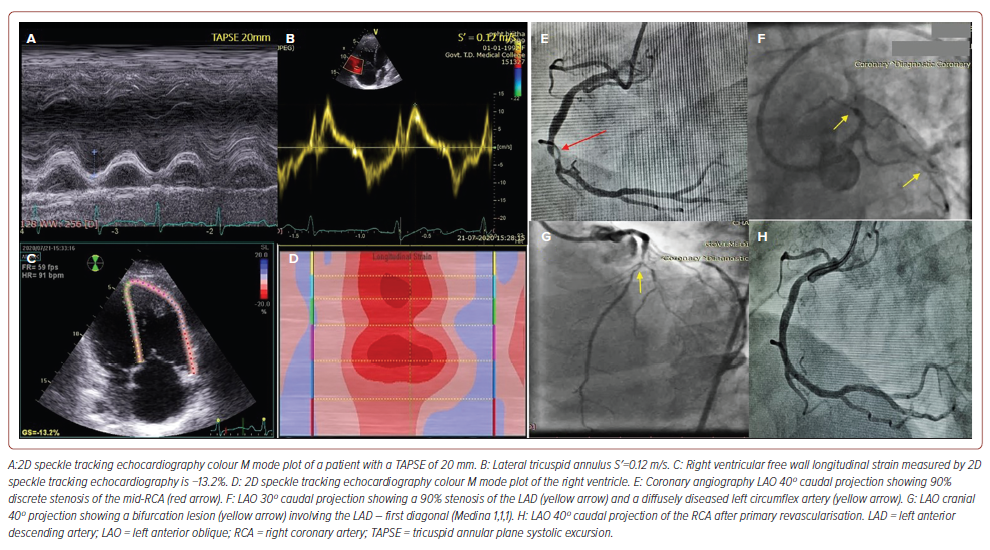
The clinical application of impaired RVfwLS with normal TAPSE and S′ in a patient with multivessel disease is shown in Figure 2. In stable patients presenting ECG evidence of RV MI and apparent normal RV function based on conventional parameters (TAPSE, S′, RV FAC), a low RVfwLS may indicate an impending risk of RV failure and mandates vigilant monitoring. Quantification of the longitudinal displacement of the RV free wall may serve as a valid surrogate for comprehensive assessment of RV function, considering that longitudinal fibre shortening accounts for approximately 80% of RV mechanics.11 Despite this, TAPSE solely quantifies the spatial displacement of the lateral tricuspid annulus towards the echocardiographic probe, rendering it significantly sensitive to angular discrepancies. Furthermore, TAPSE lacks the specificity to distinguish between the active contraction of the RV myocardium and the passive displacement of the RV free wall, which occurs as a consequence of LV contraction and the systolic interaction between the RV and LV. Consequently, TAPSE may inaccurately amplify the assessment of RV function in pathological states characterised by afterload incongruity, such as pulmonary hypertension, tricuspid regurgitation and the early phase of cardiac decompensation.
Consideration of cardiovascular comorbidities is crucial due to their potential impact on longitudinal strain measured by 2D STE, particularly their effect on loading conditions affecting both LV and RV strain. Patients with high afterload, such as those with systemic hypertension and pulmonary hypertension, may have lower LV and RV longitudinal strain.10 For example, increased systemic pressures due to sympathetic activation can reduce LV global longitudinal strain by altering afterload. However, in cases of inferior wall infarction, pulmonary pressures typically remain below 20 mmHg unless concurrent pulmonary hypertension exists, minimising the impact of loading conditions on RVfwLS. The exclusion of the interventricular septum in calculating RVfwLS eliminates the influence of left-sided heart diseases on RV strain assessment. Moreover, thick pericardial fat pads in elderly individuals (age >65 years) may impede the precise identification of endocardial borders, resulting in falsely low values of RVfwLS.8 Although in the present study a notable percentage of diabetic (60%) and hypertensive patients (70%) had low RVfwLS, the observed differences between the groups with low RVfwLS and normal RVfwLS were not significant (diabetes, p=0.061; hypertension, p=0.107). Understanding the influence of cardiovascular comorbidities on longitudinal strain is essential for interpreting RVfwLS in various clinical conditions.
Low RVfwLS in individuals with normal conventional echocardiographic parameters identifies a subset of patients that could be associated with major adverse cardiovascular outcomes, facilitating advanced risk stratification. The demonstrated correlation between impaired RVfwLS and angiographic TVD provides a basis for more personalised therapeutic approaches in managing complex coronary artery conditions in IWSTEMI patients. RVfwLS serves as a critical tool in discerning elevated arrhythmic risk among IWSTEMI patients, supporting the implementation of precise and proactive intervention strategies. Vigilant monitoring of RVfwLS in the aftermath of revascularisation is essential for the timely detection of any decline in cardiac function, thus enabling early intervention to avert MACE.
Limitations
The limitations of this study encompass its single-centre, prospective and observational nature. Although the association between RVfwLS and MACE could be explained, in part, by the mechanisms mentioned earlier, an effect of cardiovascular comorbidities on these variables could not be entirely excluded. In addition, it is imperative to note that the reference values for RVfwLS were derived from a non-peer-reviewed source, introducing a potential limitation to the study. However, it is of note that our derived RVfwLS cut-off value of −26.63 ± 5.06%, established from a pilot study of 75 healthy subjects, aligns closely with internationally recognized normal values17, providing external validation to our findings.
Conclusion
Our study reinforces the value of RVfwLS as an adjunct to traditional echocardiographic methods. RVfwLS is notably effective in identifying patients at increased risk of arrhythmias and right heart failure, underscoring its role as a significant prognostic marker for these adverse outcomes and highlighting its importance in guiding patient management and therapeutic interventions.
Clinical Perspective
- Right ventricular free wall longitudinal strain (RVfwLS) could predict heart failure and arrhythmias in patients with inferior wall ST-elevation MI.
- RVfwLS could identify patients with subclinical RV dysfunction at risk of major adverse cardiac events, aiding risk stratification.
- RVfwLS correlates with angiographic characteristics and could serve as a predictor of multivessel disease and Type C lesions.










