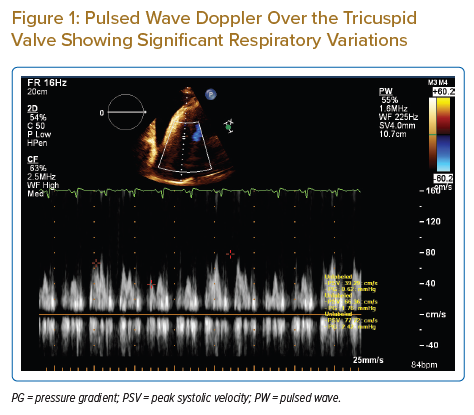
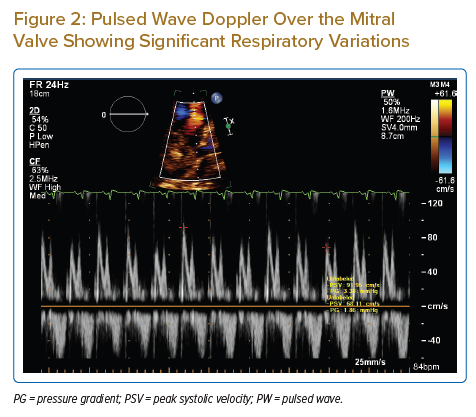
Sarcoidosis is a granulomatous disease of unknown aetiology, the pathological hallmark of which is non-caseating granulomas. Sarcoidosis can affect various organs, including the heart.1 Cardiac involvement by sarcoidosis (CS) can affect any portion of the heart, including the pericardium, atria, ventricles, papillary muscles and valves. Clinical presentation of CS varies widely, from asymptomatic to decompensated heart failure, heart block, and malignant arrhythmias.2 CS has various phenotypes in cardiac imaging with either ventricular septum thinning, especially in the basal portion, ventricular wall thickening, dilatation, regional wall motion abnormalities, valvular dysfunction or, rarely, pericardial effusion. This makes the diagnosis of CS challenging in many clinical settings.3
Case Report
A 65-year-old female known to have type 2 diabetes, on oral hypoglycaemic medications with no other significant past medical history, presented with a 2-week history of the gradual onset and progressive course of dyspnoea, orthopnoea, dry cough and lower limb oedema, as well as reduced appetite and lethargy. She had no other cardiac complaints and was admitted to another hospital. Clinically, the patient had signs of cardiac tamponade and echocardiography revealed a large pericardial effusion with echocardiographic signs of increased intrapericardial pressure. An emergency pericardiocentesis was performed and the patient was discharged after 3 days, following improvement of her symptoms and follow-up echocardiography that revealed minimal pericardial effusion. One week later, the patient presented to King Fahd Military Medical Complex (Dhahran, Saudi Arabia) with recurrence of her initial symptoms. The physical examination revealed a low-bodyweight female lying in a semiseated position with respiratory distress, tachypnoea, tachycardia (heart rate 120 BPM), hypotension (blood pressure 80/40 mmHg) and engorged jugular veins. Local cardiac examination revealed distant heart sounds and decreased breath sounds at both lung bases. Urgent echocardiography revealed a large circumferential pericardial effusion with echocardiographic signs of cardiac tamponade. Urgent pericardiocentesis, with a drain of approximately 1.5 l, was performed.
Investigations
ECG showed sinus tachycardia, left axis deviation, low voltage and poor R wave progression on precordial leads. Initial echocardiography showed normal left ventricle systolic function, large pericardial effusion with signs of increased intrapericardial pressure (Supplementary Material Video 1 and 2; Figures 1 and 2). Pericardial fluid analysis showed clear serous fluid, where cells were mainly lymphocytes, remarkable exudative effusion, with no malignant cells, and negative for acid-fast bacillus and tuberculosis polymerase chain reaction. The CT coronary angiogram showed non-significant coronary artery disease. A CT of the chest showed bilateral symmetrical hilar mediastinal lymphadenopathy and bilateral pleural effusion. Lymph node (LN) biopsy showed a picture of non-necrotising granulomatous lymphadenitis. Cardiac MRI (CMR) was performed 3 months after the acute illness because of its unavailability at time of hospitalisation as a result of the COVID-19 pandemic. CMR revealed diffuse patchy epicardial late gadolinium enhancement (LGE) at the basal to mid-inferior, inferolateral and lateral myocardial walls with patchy mesocardium at the basal septum.
Differential Diagnosis
The differential diagnosis of this condition includes myopericarditis, cardiac sarcoidosis, tuberculosis and lymphoma.
Management
After the second pericardiocentesis and a review of the chest CT findings of with the thoracic surgeon in a multidisciplinary team discussion, the decision was made to undertake video-assisted thoracic surgery. Biopsies were was taken from the mediastinal LN and the pericardium; a pericardial window was also performed. The patient was then started on oral corticosteroid therapy 25 mg once daily, ibuprofen and colchicine, and was discharged home to be followed up as an outpatient.
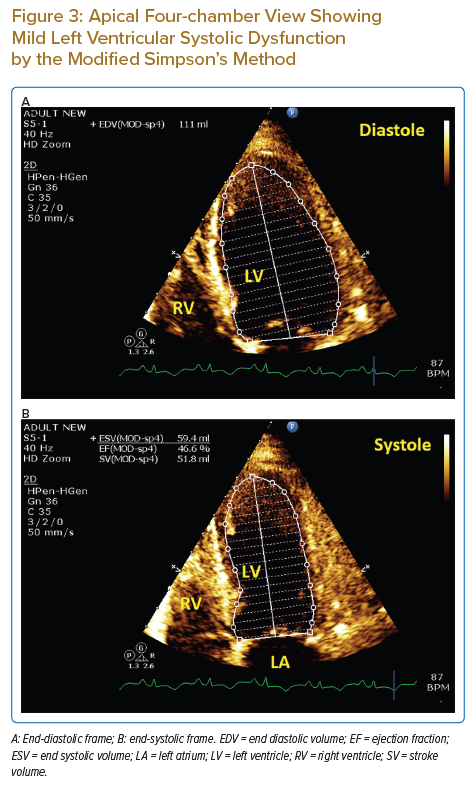
However, 5 days after discharge, the patient presented with anuria and shortness of breath. Her initial laboratory work-up revealed severe metabolic acidosis and acute renal injury. She was readmitted to the intensive care unit. A urogenital ultrasound did not reveal any obstruction. The urology team’s assessment was that the underlying cause was acute urine retention, secondary to urogenic autonomic bladder. A urinary catheter was placed and the patient’s condition improved.
During the intensive care unit admission because of acute kidney injury, echocardiography revealed mild left ventricular systolic dysfunction and new regional wall motion abnormalities in the form of hypokinesia and ballooning of the apical segments; other segments were hyperkinetic (Figure 3; Supplementary Material Video 3, 4 and 5).
A few days after resolution of metabolic acidosis and normalisation of kidney function tests, echocardiography was repeated and revealed normal left ventricular systolic function and normal regional wall motion (Supplementary Material Video 6 and 7).
Discussion
This report presents a rare case of cardiac sarcoidosis with recurrent large pericardial effusion causing tamponade. Echocardiography played an important role in establishing this diagnosis; the significant respiratory variations in wave Doppler over tricuspid and mitral valves provided an indication of increased intrapericardial pressure and, in the clinical context of distress and haemodynamic instability, mandated the rapid performance of pericardiocentesis. The recurrence of the large pericardial effusion after 1 week is also a rare finding, reported in very few cases.4,5 Believing in the importance of investigating unexplained pericardial effusions, especially if large, symptomatic and recurrent, we undertook many investigations. Laboratory results did not help in our search for the specific aetiology of pericardial effusion in our patient. However, the presence of mediastinal lymphadenopathy and histopathology of the LN biopsy were major factors in establishing the diagnosis. The absence of significant coronary artery disease by cardiac CT helped rule out underlying coronary artery disease. The CMR findings were non-specific; this could be attributed to the lateness (after 3 months) of the imaging or due to the nature of the disease and heterogeneity of the LGE affection pattern.6,7 During the clinical course, the patient developed acute renal shut down and severe metabolic acidosis; echocardiography showed a picture of stress-induced cardiomyopathy with transient ballooning of the apical segments, which improved to normal after 2 days. This is rare and, to the best of our knowledge, has not been reported before in such a clinical context.
Follow-up
During clinical follow-up 2 months later, the patient showed good improvement of her symptoms and complete recovery of acute renal failure. The patient was on steroids for 2 months, which were gradually tapered over 4 weeks, and she has remained symptom-free. The patient also had elevated blood glucose levels, for which she was started on insulin.
Conclusion
Pericardial effusion is a rare presentation of cardiac sarcoidosis and can be not only large enough to cause cardiac tamponade, but also recurrent. Stress-induced cardiomyopathy occurring in such a clinical context has not been reported previously.