Heart failure is a leading cause of death and morbidity worldwide.1 It has a protean clinical history punctuated by recurrent hospitalisation after the initial diagnosis that is characterised by progressive deterioration in the cardiac structural and functional status of patients without interventions.2
Heart failure mortality remains high, despite the advancement in treatment modalities.3 Pharmacological treatment of heart failure has significantly reduced this burden over the past three decades.4,5 Demonstration of the effects of angiotensin-converting enzyme inhibitors (ACEI) in reducing heart failure hospitalisation and mortality among patients with heart failure with reduced ejection fraction (HFrEF) in the 1980s started a pharmacological revolution.6–8 This was followed by β-blocker and mineralocorticoid receptor antagonist (MRA) trials in the 1990s. Composite analysis of these trials showed that the simultaneous initiation of these drugs in patients with HFrEF derived early benefits during treatment.9 This has contributed to the change in the pharmacological treatment strategy of HFrEF.
Recently, two new classes of drugs – angiotensin receptor–neprilysin inhibitor (ARNI) and sodium–glucose transporters 2 inhibitors (SGLT2I) – were also shown to have independent additive benefits in regard to major heart failure outcomes.10–14 The independent effect of ARNI compared with enalapril led to the change in guideline recommendation of ARNI as either a Class I recommendation in HFrEF over ACEI, or ARNI as the alternative if ACEI is clinically contraindicated.2 In contrast, SGLT2I have been shown to reduce heart failure hospitalisation and heart failure death in both heart failure patients with or without diabetes, but interestingly, they have effects across all spectrums of the ejection fraction.11-15 This essentially means that SGLT2I, unlike other foundational heart failure treatment classes, can now be commenced in all heart failure phenotypes without ejection fraction (EF). ARNI and SGLT2I have now been added on the expanded list with the other classes of heart failure drugs that now make up the four foundational classes in the treatment of HFrEF: β-blockers, ACEI/ARNI/angiotensin receptor blockers, MRA and SGLT2I.
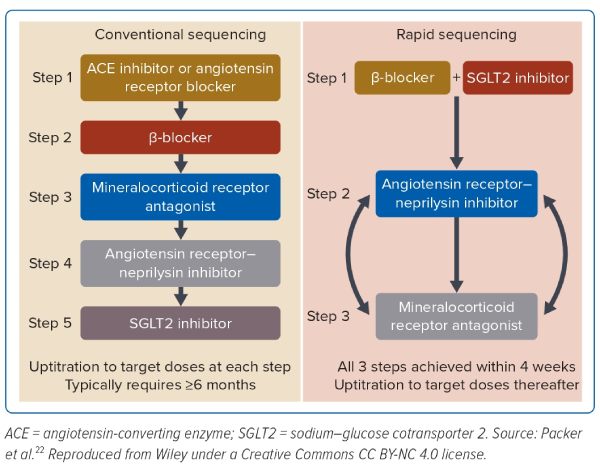
The treatment strategy adopted over the past decades has recently come under scrutiny with the development of new classes of drugs and decades of pharmacological experience in the management of HFrEF.9 Today, there is a paradigm shift in the heart failure community from the traditional sequential treatment strategy to the simultaneous strategy in HFrEF. The traditional strategy entails the ‘sequential’ initiation of the foundational drugs: starting with the lowest dose of β-blockers, escalated over days and weeks until the maximal tolerable dose is reached, then followed by an ACEI or angiotensin receptor blocker if ACEI is intolerable, or even better, ARNI with the lowest dose and escalated over days and weeks until the maximal tolerable dose is reached. This approach is repeated with the MRA, as indicated according to the guideline’s recommendations.
The sequential treatment strategy takes up to a period of 6 months, with multiple clinic visits. It was based on many assumptions and not on evidence. First, it is a recapitulation of when the drugs were discovered in heart failure treatment, and second, it is impractical to employ in contemporary practice, as there are frequent reviews for optimisation over a 6-month period. The benefits are, however, seen early with even low doses, and there is no rationale to achieve optimal dosages of one class before the addition of another over that period. In fact, the composite analysis of older heart failure trials has shown early benefits after initiation of all treatments.16
Additionally, the composite analysis of the trials in SGLT2I showed that patients treated with ARNI derived the same benefits as those without ARNI, suggesting that treatments can be initiated simultaneously.15,17–21 Therefore, the current recommendation is to adopt a ‘simultaneous’ initiation strategy. This strategy is known to harness the beneficial effects of all foundational heart failure drug treatments simultaneously and to reduce the duration of optimisation to 3 months.22,23 It is, therefore, crucial to understand these changes and adopt them into the contemporary clinical practice to accord HFrEF patients optimal evidence-based management.
Pharmacological treatment is readily available and accessible globally to both resource-rich and resource-limited countries. It can be prescribed by any physician who has an interest and expertise in heart failure management, especially in practices where heart failure expertise is not available. In Papua New Guinea (PNG), a low-to-middle-income country of 13 million people with an estimated high incidence and prevalence of heart failure, pharmacological evidence, changes and application in the treatment of HFrEF appears critically important in clinical practice, as it is the only available treatment modality for the management of HFrEF in the country.
This review highlights the recent definition of heart failure, the addition of the new classes of heart failure drugs and the changes in the treatment strategies with the intention of highlighting the gaps in heart failure management in PNG, and how these gaps could be addressed.
Definition of Heart Failure
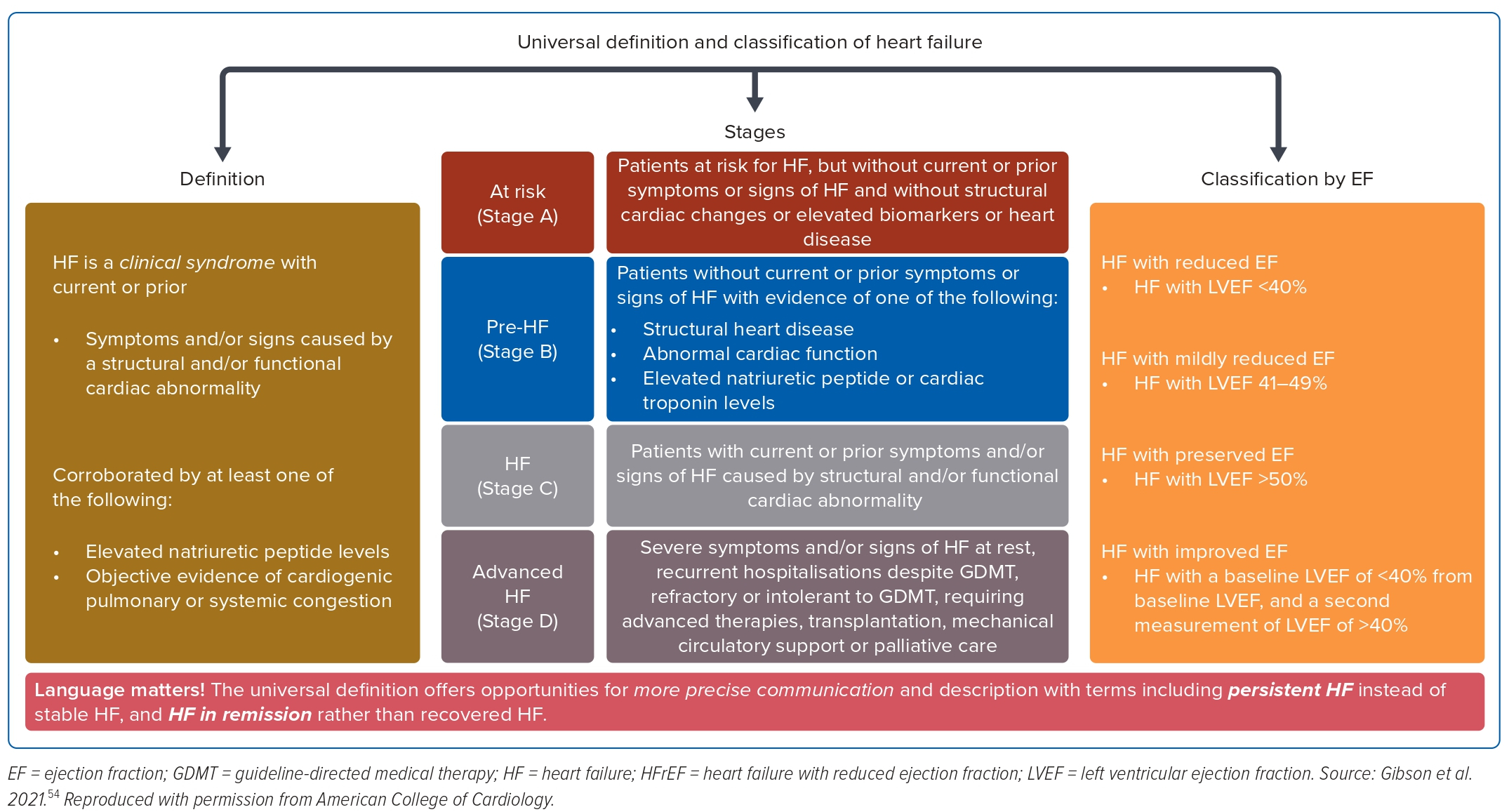
Universal Definition and Classification of Heart Failure
Heart failure is not a single pathophysiological entity, but a cluster of clinical symptoms (breathlessness and fatigue) and signs (raised jugular venous pressure, fine lung crackles and lower leg oedema) associated with evidence of structural and or functional cardiac abnormality that results in reduced cardiac output to meet its metabolic demands at rest or during exertion.2
Many terminologies were used to describe heart failure prior to 2021, and they were based on anatomy, such as right or left heart failure; on haemodynamic shifts, such as the forward or backward failure; and on the duration of symptom manifestations frequently termed as acute or chronic heart failure. Although the acute and the chronic heart failure terminologies are retained in the contemporary practice to facilitate escalation or de-escalation of decongestive treatment, the other two do not hold any therapeutic merits.
In 2020, three professional heart failure organisations (Heart Failure Society of the American College of Cardiology, Heart Failure Society of European Society of Cardiology and the Japanese Heart Failure Society) proposed a consensus definition of heart failure, its diagnostic criteria and phenotypical classification based on the EF.24 The objective is to standardise and harmonise the terminology globally, and simplify the relevant clinical diagnosis and management.
Diagnosis of Heart Failure
Heart failure diagnosis according to the consensus definition is based on the triad of the objective clinical findings of: clinical symptoms and signs of heart failure (Supplementary Table 1), evidence of structural and or functional abnormalities (ECG, chest X-ray, echocardiography and other imaging) suggesting heart failure, and raised natriuretic peptide.
Classification of Heart Failure
The phenotypical classification of heart failure is based on the EF.24 The EF is the contemporary surrogate for all clinical decisions and prognostication; for example, the decision for the consideration of device therapy is based on EF ≤35%, and that is also the threshold for the initiation of medical therapy, such as MRAs, in the traditional sequential strategy.25
Additionally, lower EF is prognostically associated with poor quality of life and higher heart failure mortality.26 Therefore, EF is important in the classification of heart failure with four classes:
- HFrEF (EF ≤40);
- heart failure with mildly reduced EF (HFmrEF; EF 41–49%);
- heart failure with preserved EF (EF ≥50%); and
- heart failure with improved EF (EF ≤40% on baseline and second EF >40% after treatment).
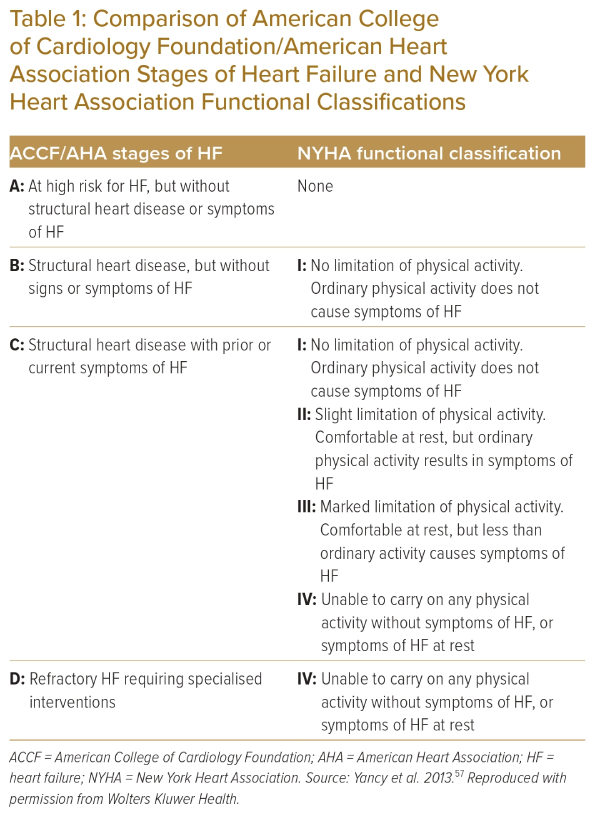
The American Heart Association classification of heart failure attempts a descriptive staging of risk factors and the symptomatic nature of heart failure, as shown in Table 1, and the combined definition and classifications shown in Figure 1.
Heart Failure Treatment
Mechanisms of Heart Failure
A few theories were postulated as possible underlying mechanisms of the progression of heart failure before the trials of the neurohormonal modulators in the 1980s. One such theory was the haemodynamic theory, a traditional concept where any insults to the heart are detected by a drop in blood pressure followed by renal blood flow. Subsequent haemodynamic changes set in to maintain perfusion, which ultimately lead to salt and water retention. Peripheral vasoconstriction and increasing preload exacerbate heart failure.27 Treatments based on the haemodynamic model, such as bed rest, inactivity, fluid restriction, digitalis and vasodilators, have not proved beneficial.28–31 Furthermore, the inotropic drugs used to improve contractility resulted in dismal outcomes.32–34
The other theory was the peripheral model theory, whereby, a specific cardiac failure-related myopathy develops because of poor muscular perfusion or the impacts of inflammatory cytokines and neurohormonal activation, or perhaps lack of fitness. The deconditioning of the skeletal muscles from these processes results in weakness and fatiguability.27 This theory may be a contributing factor, but does not appear to be the archetype pathology of heart failure.
The neurohormonal model gained traction in the late 1980s, when the ACEI trials demonstrated the beneficial impacts of countering the renin–angiotensin–aldosterone system.6–8 Further trials of β-blockers followed by MRAs have unequivocally established neurohormonal activation as the underlying mechanism perpetuating heart failure. That era is often called the ‘era of pharmacotherapy’ in heart failure management.
The Role of Neurohormonal Modulation in Heart Failure
The neurohormonal mechanism in heart failure was initially thought to be merely a compensatory mechanism, but that began to change in 1983, when Peter Harris’ paper began to question the role of the mechanism.35 He argued that the neurohormonal stimulation noted among heart failure patients was a natural response to low heart function that becomes deleterious in chronic heart failure. In fact, it was the mechanism perpetuating heart failure.36 To prove this, Harris and Robert Farrari went to India to test their hypothesis, because it was difficult in western patients. Several observational studies among severe heart failure patients in India showed that the neurohormonal activation mechanisms in heart failure were indeed deleterious and were perpetuating heart failure.37–39
The Consensus Study, although a small study by today’s standard, with 127 patients with chronic advanced HFrEF, was the first ACEI trial to show significant clinical improvement and reduction in mortality.7 This was followed by the SOLVD among less symptomatic patients, also showing significant reduction in heart failure-related deaths and hospitalisation.8 Further meta-analyses of ACEI trials have established the beneficial impacts of the ACEI, thus establishing the renin–angiotensin–aldosterone system as a neurohormonal pathway that confers deleterious effects in heart failure.
The sympathetic activation noted in heart failure is initially beneficial to improve contractility and maintain perfusion. However, it has long-term deleterious effects, as shown in Supplementary Figure 1. It was initially conflicting to initiate β-blockers to negate what was thought to be a helpful compensatory mechanism. The initial experience with β-blockers was reported in 1976, and its mortality benefit was reported in 1977.40,41 However, the first randomised controlled trial was published in 1993, showing mortality benefits, reduction of hospitalisation and improved quality of life.42 It was not until after 1997 that the β-blockers were formally approved to be used in combination guideline-directed therapy among patients with HFrEF.
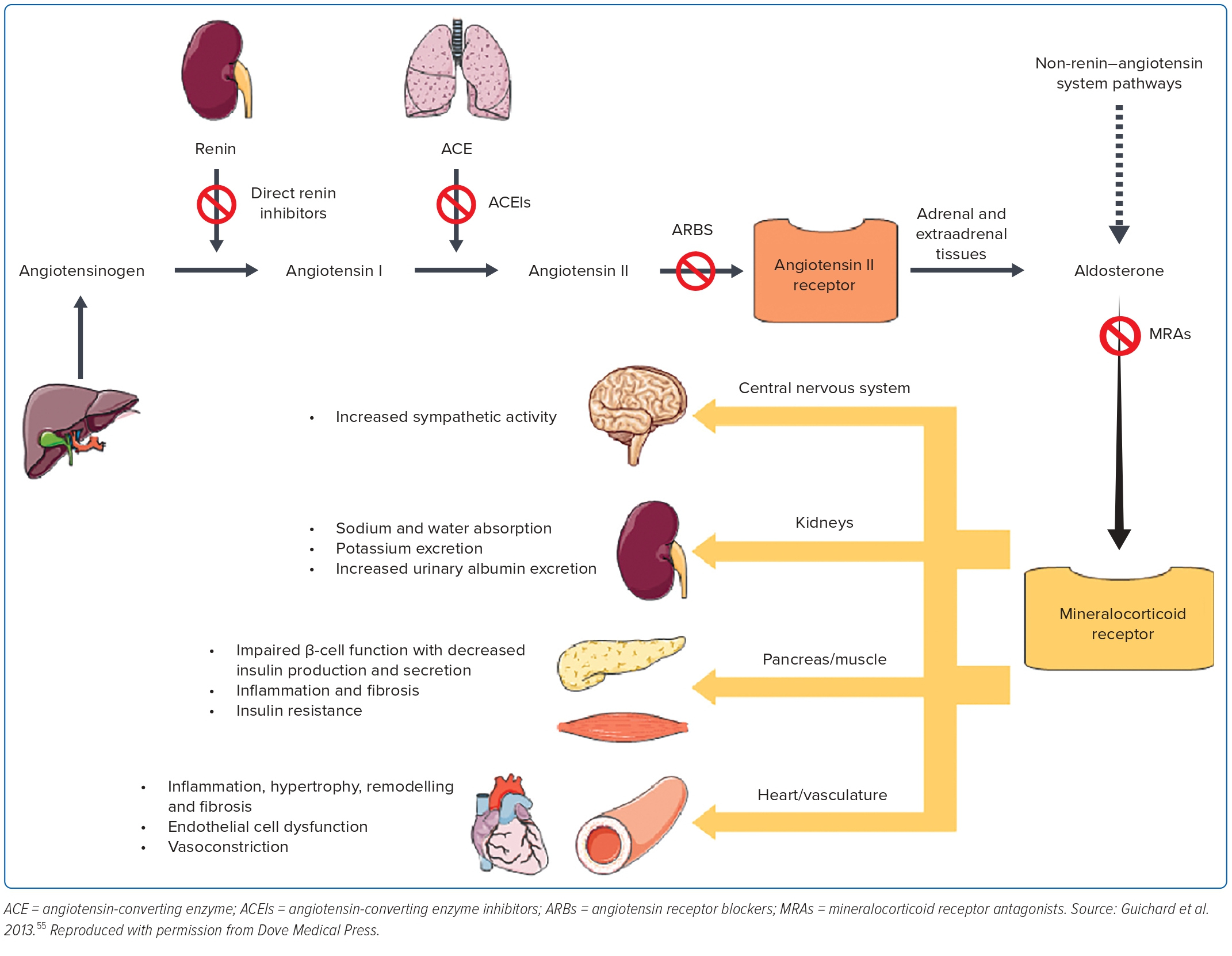
Despite the use of ACEI in blocking the renin–angiotensin–aldosterone system, it was becoming increasingly clear that the aldosterone system was not blocked adequately. This led to the design of the RALES trial to assess the efficacy of spironolactone, a non-selective aldosterone antagonist among patients with advanced HFrEF (EF ≤35%). It shows a 35% reduction in all-cause mortality, 35% lower rate of heart failure-related hospitalisation and 41% symptoms improvement compared with the placebo.43
This was followed by the EPHESUS trial to offer the more selective aldosterone blocker, eplerenone, to reduce the 10% of gynaecomastia noted in the RALES trial. It shows a reduction in all-cause mortality, heart failure-related deaths and heart failure-related hospitalisation. Although, gynaecomastia was very low and comparable with the placebo, hyperkalaemia was significant among those who received eplerenone.44 These trials have unequivocally shown that antagonising the aldosterone activation (Figure 2) in heart failure further offers benefits in addition to standard treatment in HFrEF.
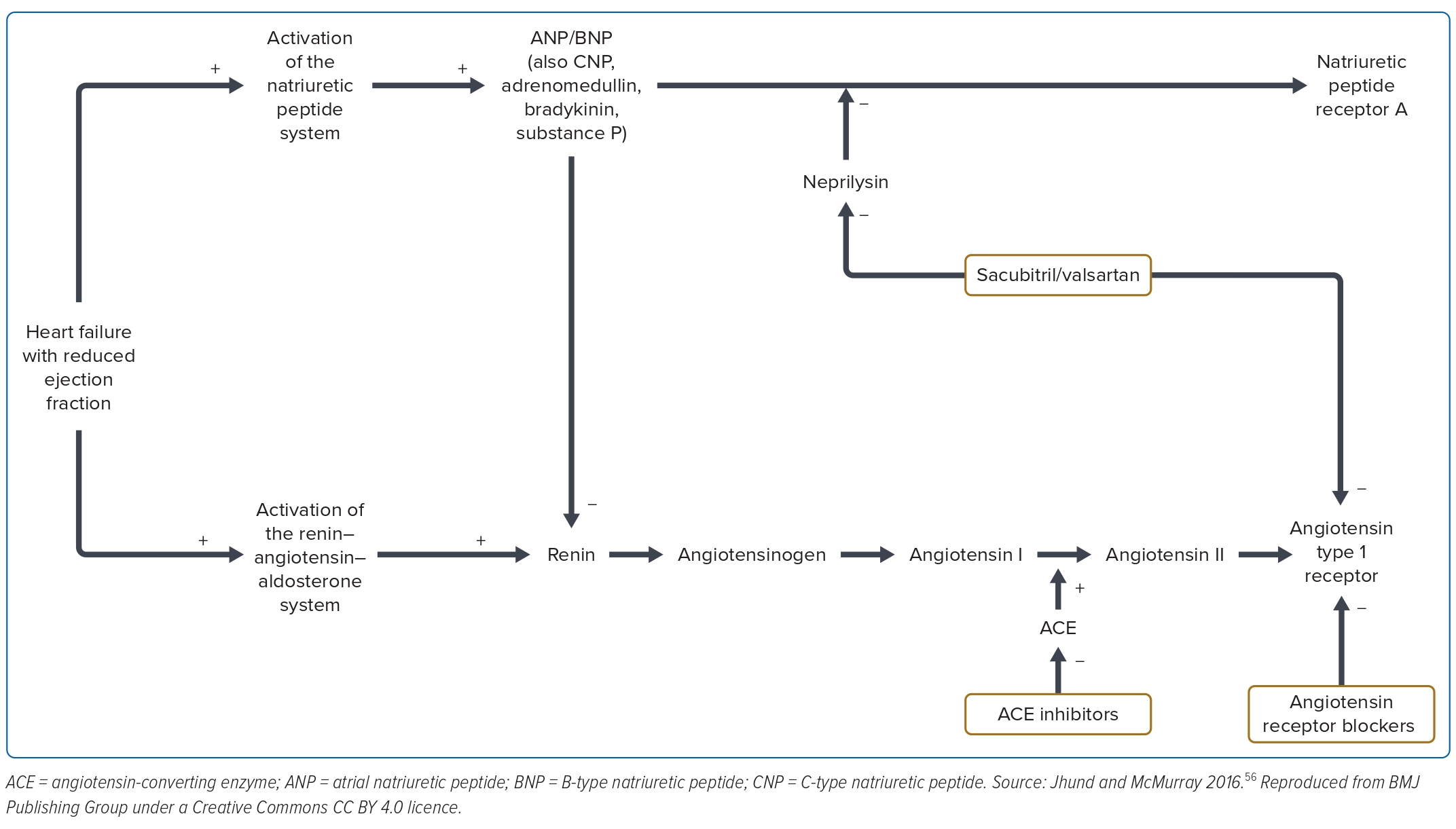
Vasoactive substances are known to be crucial in counteracting the devastating pathophysiology of the heart failure, as shown in Figure 3. Attempts to give exogenous vasoactive substances and or isolated neprilysin inhibition were not successful.45-47 Dual inhibition of neprilysin and ACE with omapatrilat – a neprilysin and ACEI – was not effective in reducing the primary endpoints of mortality and heart failure hospitalisation, but improved the secondary outcomes with a high rate of angioedema in the omapatrilat group.48 This was due to the inhibition of aminopeptidase P by omapatrilat. In the PARADIGM trial, sacubitril/valsartan – a neprilysin inhibitor – was compared with enalapril in the background of standard treatment among the ambulatory HFrEF patients. It demonstrates significant reduction in deaths and heart failure hospitalisation compared with enalapril.2
These historical trials have established neurohormonal activation as the predominant underlying pathophysiological mechanism of heart failure. There are four distinctive pathways with the renin–angiotensin system and the aldosterone system closely intertwined, and therefore, are often taken as one system: the renin–angiotensin–aldosterone system, the sympathetic system and the natriuretic peptide system.
History of Treatment of Heart Failure
The pharmacological treatment of heart failure before the 1980s was anecdotal and based on assumed pathophysiology. Bed rest, diuretics and vasodilators were the mainstay of treatment. Although β-blockers were the first group to show mortality benefits, it was not until the 1990s when clinical trials were conducted (Supplementary Table 2) showing the beneficial effects in HFrEF and finally accepted in 1997 as a class of treatment for HFrEF.41
Instead, it was the ACEI trials in the late 1980s that ushered in the neurohormonal mechanism as the underlying cause of heart failure deterioration, and countering this mechanism has led to improved clinical outcomes.6 This was further supported by evidence from the β-blocker trials countering the sympathetic activation, and the MRA trials countering the aldosterone activation.35,36,43,44
The recent trials of ARNI and the SGLT2I classes of heart failure treatments have further broadened the pathophysiological mechanisms, and have had significant heart failure benefits in addition to standard treatments in patients with HFrEF.2,11-14,21
Angiotensin-converting Enzyme Inhibitor
ACEI was the first class of heart failure treatment shown to reduce all-cause mortality, cardiovascular (CV) mortality, heart failure hospitalisation and improve heart failure symptoms among patients with HFrEF (Supplementary Table 3). It forms the backbone of heart failure treatment unless the patient is intolerant and/or side-effects ensue, and, in such situations, sacubitril/valsartan is recommenced after a 36-hour washout period.2 The recent ESC 2021 guideline recommends the use of ACEI as Class IIb for HFmrEF.19
β-blockers
Trials of β-blockers (Supplementary Table 2) have shown that, counteracting the chronic sympathetic activation in heart failure patients reduces CV death, hospitalisation and improves symptoms. They are an integral component of the foundational therapies for treatment of HFrEF and are recommended to be started together with ACEI with no preferences over another. With the new treatment strategy of simultaneous initiation of the foundational drugs, the timing of initiation has become irrelevant. The 2021 European Society of Cardiology heart failure guideline recommends the use of β-blockers in HFmrEF as Class IIb indication.19
Mineralocorticoid Receptor Antagonists
The MRA class of antifailure drugs (eplerenone, spironolactone) has been trialled in studies among patients with heart failure ranging from mild to severe HFrEF, and they have been shown to reduce CV deaths and heart failure hospitalisation with standard treatments (Supplementary Table 4).43,44 MRAs were therefore recommended to be started in HFrEF patients with EF ≤35% after 3 months of optimal treatment in the older sequential treatment strategy. However, it has now been recommended that MRAs be initiated with other standard treatments of HFrEF in the simultaneous strategy. The recent European Society of Cardiology guideline recommends MRAs to be used in HFmrEF patients as Class IIb.19
Angiotensin Receptor–Neprilysin Inhibitor
Sacubitril/valsartan reduces heart failure death, all-cause mortality and heart failure-related hospitalisation. This has been demonstrated by the PARADIGM-HF trial. This was a prospective randomised, double-blind study with 8,399 patients with HFrEF (≤40%) in New York Heart Association II–IV comparing neprilysin inhibitor, sacubitril/valsartan (LCZ2696), with enalapril (ARNI versus enalapril ACEI). Sacubitril/valsartan was superior to enalapril in reducing CV mortality and hospitalisation, and was associated with a significant reduction in the N-terminal prohormone of brain natriuretic peptide among the ambulatory patients with HFrEF.2 Mortality reduction was more pronounced among the early initiators than the late initiators. This suggests that this class of drug therapy can be initiated early in the treatment of heart failure rather than later. The other effects are improvement of quality of life, reduction in the need for diuretic therapy and a reduction in the incidence of insulin therapy in diabetes patients.
Patients being considered for ARNI must have an optimal blood pressure, fewer signs of decongestion to allow for tolerance, estimated glomerular filtration rate ≥30 ml/min/1.73m² and have met the washout period of 36 hours to prevent angioedema. ARNI is now recommended as a first line therapy for ambulatory patients with HFrEF and as a Class IIb indication for HFmrEF.19
Sodium–Glucose Cotransporter 2 Inhibitor
SGLT2I is a new class of drug that has been shown to reduce CV deaths, all-cause mortality, hospitalisation, improve physical function and quality of life, and stabilise the decline in renal function.11,12 Meta analysis of the DAPA-HF and EMPEROR-Reduced trials showed no heterogeneity in the CV mortality.49
The EMPULSE trial showed that empagliflozin started in patients with acute heart failure reduces CV mortality, morbidity and recurrent hospitalisation.50 Therefore, SGLT2I is recommended to be initiated in acute heart failure. The recent DELIVER trial showed that dapagliflozin significantly reduces CV mortality, morbidity and hospitalisations outcomes across all spectrums of the EF. In fact, the maximal benefit was noted among patients with higher EF.14
The European Society of Cardiology heart failure guideline in 2021 was released before the announcement of the DELIVER trial and, therefore, SGLT2I was not recommended as a Class I treatment, but rather, as a Class IIA treatment. Inevitably, it will be designated as a Class I drug in the next guideline.
The American College of Cardiology guideline, however, was released in 2022 after the DELIVER trial and, therefore, included SGLT2I as a Class I treatment for all phenotypes of heart failure. It is, therefore, the only foundational drug that does not require prior echocardiography to be initiated, and will be commenced on all heart failure phenotypes prior to echocardiography, except in patients who have had ketoacidosis, dehydration, acute illness and those with estimated glomerular filtration rate <20 ml/min/1.73m2. Supplementary Table 5 summarises the SGLT2I trials.
Treatment Strategies
There has been a recent shift in the treatment strategy of HFrEF from the sequential initiation of heart failure drugs to the simultaneous initiation (Figure 4). This shift has been brought about by the following factors: the early benefits of individual heart failure drugs and the unrealistic duration of treatment optimisation in the contemporary practice.
Although the clinical drug trials in heart failure have had targets to achieve ‘target doses’ in their protocol, nearly half of all patients in these trials have not achieved these targets.50 These were due to intolerances, adverse effects and trial protocol restriction; for example, hyperkalaemia.47 Despite the suboptimal target doses, patients still derived significant heart failure benefits. The composite analysis of all these trials further showed that patients with HFrEF derived benefits from the individual drugs early in their treatments.
Regular clinical reviews of heart failure patients over a minimum of a 6-month period as it has been in the sequential strategy is no longer deemed feasible in contemporary practice. These have, therefore, led to the change of treatment strategy from the traditional sequential strategy, where the foundational heart failure drugs are escalated to target doses over 6 months, to the new simultaneous sequencing strategy, where the foundational drugs are initiated simultaneously with the objective of achieving the target doses within 4 weeks.22,23
Status of Heart Failure and its Treatment in Papua New Guinea
Heart failure is not registered as a specific entity in the National Department of Health database and, therefore, it is not documented as a specific cause of morbidity and/or mortality in the National Department of Health statistics. However, the risks of heart failure are registered as separate entities.
The National Department of Health mortality and morbidity record in 2016 showed ischaemic heart disease as the fourth leading cause of death (unpublished). In fact, Urarang et al. showed that ischaemic heart disease was one of the emerging causes of death in PNG.51 Furthermore, a systematic review showed a rising trend of the CV risk factors in PNG since the 1980s.52 There is, however, no literature on adult heart failure incidence and prevalence among the PNG population. A historic regional observation in Mount Hagen showed cor pulmonale and rheumatic valvular heart disease as the first and second commonest causes of heart failure.53 With limited literature, it is difficult to discern the true status of heart failure in PNG. Therefore, heart failure presents an area of interest for epidemiological and clinical research in PNG.
The Public Pharmaceutical Board of PNG has the authority to enlist and/or delist new and old drugs on the public pharmaceutical catalogue (PPC) based on the evidence and affordability. The list of evidence-based heart failure drugs on the PPC is rather limited, as shown by Supplementary Table 6. The clinical management of heart failure throughout the country is performed by general physicians who, in their training, have scantly covered heart failure as a module. Additionally, they do not have the evidence-based heart failure drugs on the PPC to combat the rising incidences of heart failure in PNG. Therefore, the undergraduate and the postgraduate training in medicine in PNG need heart failure modules in the internal medicine curriculum. This embedment will ensure trainees in general medicine are well-grounded in their knowledge of heart failure and its application in clinical management. Moreover, the Public Pharmaceutical Board of PNG needs to urgently review the PPC to add and expand the list of evidence-based heart failure drugs to expand the pharmacological options. While these may be intermediate solutions, the interim approaches to improving care would be for general physicians to attend conferences and webinars formally organised by their society to catch up on the subject, and apply the evidence and changes of heart failure treatment at the bedside.
Conclusion
The drug therapies for HFrEF show reduced heart failure deaths, reduced all-cause mortalities and reduced hospitalisations by modulating neurohormonal activation. Newer drugs have shown new pathophysiological mechanisms and early treatment benefits.
The evidence of early benefits from the composite analysis of all heart failure trials and the unrealistic duration of follow-up of patients for the optimisation of drugs in patients with HFrEF in contemporary practice have led to the change in the treatment strategy. The heart failure drugs for those with HFrEF are now initiated simultaneously with optimisation over 4 weeks, rather than the traditional strategy of sequential therapy and optimisation over 6 months. The uptake of these developments in many countries remain dismal, and PNG, a low-to-middle-income country is no different. Understanding the evolutionary changes in this pharmacological arena of treatment of HFrEF, and incorporating them into the PNG public pharmaceutical catalogue, clinical teaching curriculum and practice will inevitably improve the outcome of patients living with HFrEF in PNG. 
Click here to view Supplementary Material
Clinical Perspective
- Heart failure (HF) definition, pathophysiology, diagnosis, classification, treatment and its strategy has changed significantly in recent years.
- The uptake of these changes globally is disparate between countries and regions, and affect management of HF patients.
- Papua New Guinea has a generalist practice where the changes in HF diagnosis, treatment and management have not been understood and adopted into contemporary practice.
- HF is managed by general physicians, with lapses in guideline-directed practice.
- Review of the National Public Pharmaceutical Catalogue with the introduction of guideline-directed HF drugs, HF training, incorporation of the changes in HF training modules and continuous professional development driven by the local physician society will help improve knowledge and practice. It will accord HF patients evidence-based care, reduce HF-related hospitalisations and mortality, and improve quality of life.