We aim to review the literature on percutaneous device closure of post-infarction VSR and, using two illustrative cases, describe a systematic planning and technical approach for performing this complex procedure.
Methods
A detailed Medline database search using the MeSH terms “ventricular septal rupture” OR “ventricular septal defect” AND “myocardial infarction” AND “septal occluder” was performed. Publications from 2002 to June 2021 were included. Only publications describing more than three cases were included, with individual case reports excluded. The search and data extraction were performed by a single reviewer (KA) with queries resolved by a second reviewer (JY).
Case 1
A 49-year-old male was planned for percutaneous closure of residual anteroseptal VSR after failed primary repair. He originally presented to another hospital with a delayed presentation for Killip class IV anterior ST-elevation MI. He underwent immediate PCI to his proximal to mid-left anterior descending artery in view of cardiogenic shock. Physical examination was significant for a pan-systolic murmur and a prompt bedside transthoracic echocardiogram (TTE) revealed a 1.2 cm anteroseptal apical VSR. An intra-aortic balloon pump was inserted and the patient was transferred to our centre for urgent surgery.
An intraoperative transoesophageal echocardiogram (TOE) revealed a 1 cm defect. A pericardial patch with multiple pledgeted sutures was used to seal the VSR from the left-ventricular (LV) side. Postoperative TTE performed on day 10 showed a residual shunt of 7 mm restrictive flow (4.73 m/s) with an oblique distance of 10–11 mm. Given persistent pulmonary oedema, the decision was made to pursue percutaneous closure.
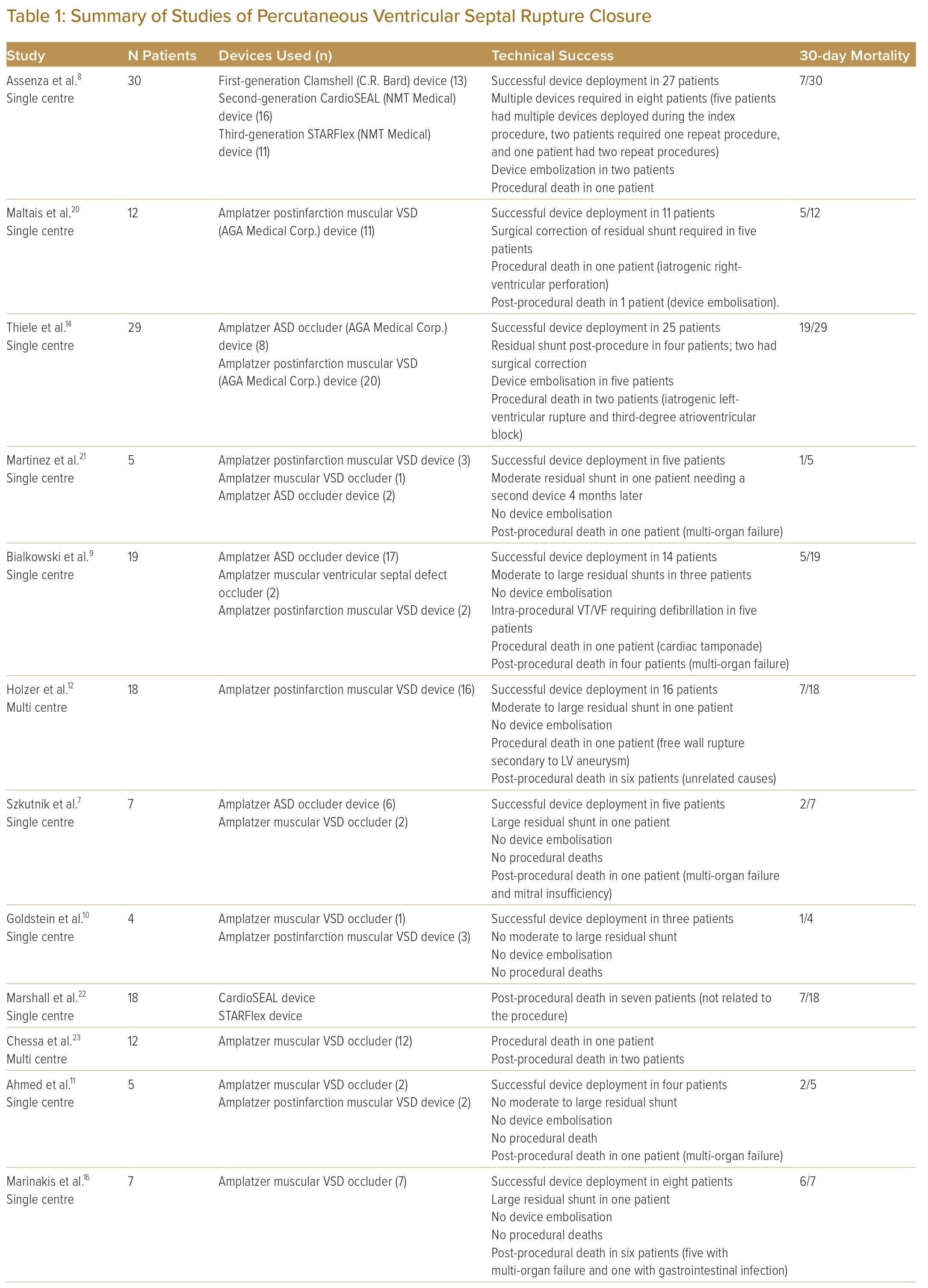
The percutaneous VSR closure procedure was performed 46 days after the initial surgery because of recurrent bacteraemia from a urinary tract infection and to allow for tissue fibrosis. A preprocedural planning CT scan showed 8–10 mm defect (Figure 1A) in the ventricular septum with a length of approximately 12 mm (Figure 1B). The procedure was performed under general anaesthesia with TOE support. TOE measured the VSR as 8 mm both on the LV and right ventricular (RV) sites. The estimated length was 9 mm with complex serpiginous morphology. Ultrasound-guided insertion of 7 Fr sheath was performed in both the right femoral vein and artery. Heparin was administered.
A right heart study was performed, and the mean pulmonary arterial pressure was elevated at 40 mmHg. There was an initial attempt to cross the VSR from the RV side, but this was not successful. This was quickly converted to a retrograde approach. The defect was crossed from the LV with a Judkin right 4 catheter and wire (Figure 1C and Supplementary Video 1). A 20 mm goose neck snare was positioned via the venous access in the pulmonary artery and the wire snared, forming an arteriovenous (AV) loop. A 7 Fr Amplazter 45° torque delivery catheter (Abbott) was advanced from the venous side of the AV loop to the LV. An 18 mm Amplatzer atrial septal occluder (Abbott) was initially deployed but there was a cobra distortion. The device was then retrieved and a smaller 12 mm muscular VSD device was deployed successfully (Figure 1D and Supplementary Video 2). The TOE showed mild residual shunt (Figure 1E and Supplementary Video 3). At 2 years follow-up, the patient was stable, with TTE showing a tiny 2 mm residual shunt.
Case 2
A 68-year-old male was admitted for delayed presentation of anterior ST-elevation MI on a background of previous intervention to the left anterior descending and left circumflex. He was initially treated medically without intervention because of significant anaemia and delayed presentation. His TTE showed an impaired LV ejection fraction of 30% and an 18 × 14 mm RV thrombus. His blood cultures were positive for group B streptococcus. The patient was treated with culture-directed antibiotics for 6 weeks and warfarin for an infected RV thrombus with septic embolisation to the lung. TTE performed after completion of antibiotic course showed resolution of the thrombus but revealed a 12 mm VSR in the muscular part of the septum. His co-morbidities and severe peripheral vascular disease precluded him from surgery. Cardiac magnetic resonance imaging revealed that all myocardial segments were viable and the calculated Qp:Qs ratio was 2.6:1, consistent with VSR with left-to-right shunting. He underwent a coronary angiogram with drug-eluting balloon therapy to his prior mid-left anterior descending artery, which showed mild in-stent restenosis.
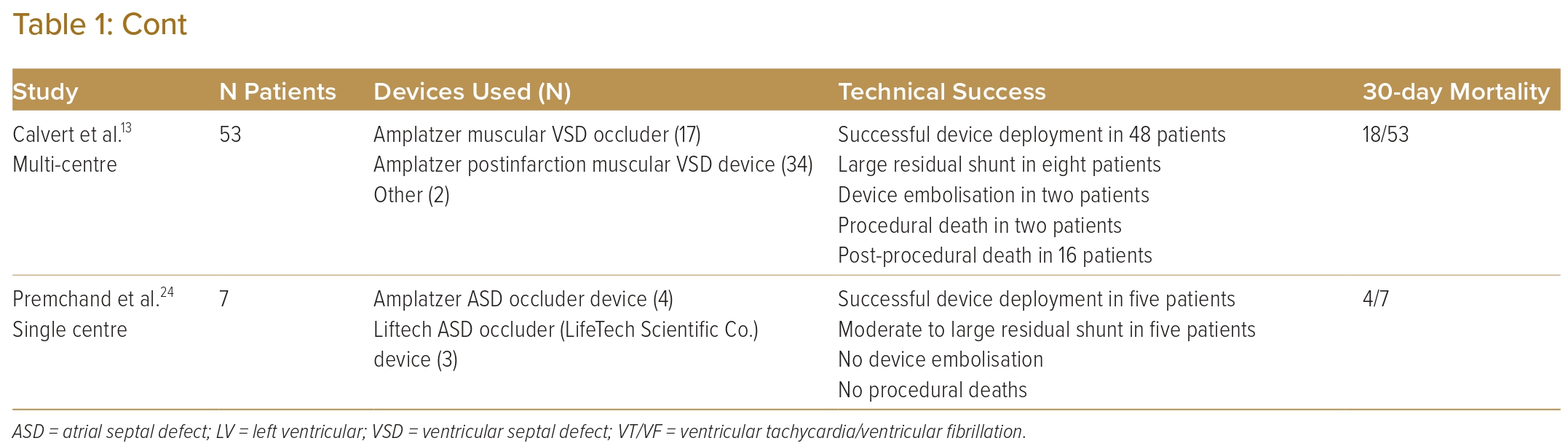
The percutaneous VSR closure procedure was performed under general anaesthesia with TOE guidance. Right-femoral venous access was obtained, and the right heart study showed a mean pulmonary artery pressure of 40 mmHg and the calculated pulmonary vascular resistance was 1.9 Wood units. As the morphology of the defect was simple and not serpiginous, the decision was made to attempt to cross the VSR from the RV side with a Glide wire (Terumo) and Gensini catheter (Balton) (Figure 2A and Supplementary Video 4). This was successful and the glide wire exchanged for a support wire. An 8 Fr Amplatzer 45° torque delivery catheter (Abbott) was then introduced into the LV over the support wire (Figure 2B; Video 5). An Amplatzer muscular VSD device (Abbott; size 16 mm) was successfully deployed with a good seal of the defect (Figure 2C and 2D; Supplementary Videos 6 and 7). The patient was discharged home well without complications.
Discussion
Considerations for Percutaneous Intervention
Anatomical factors such as site, size and morphology play an important role when considering percutaneous options. Patients with more basally located VSR after an inferior MI are less amenable for device closure because of interference from the tricuspid and mitral valve apparatus and lack of adequate tissue rim for stable implantation of the device.1 Similarly, those more apical defects may lack adequate tissue rims from the apical end. Larger defects, particularly those above 15 mm, are less likely to achieve complete closure and are vulnerable to device embolisation.7 The morphology of the defect is rarely simple, with most adopting a serpiginous shape.8 Unlike congenital VSDs, the irregularity of these defects can result in incomplete sealing and residual shunts.9
Incomplete surgical sealing or dehiscence of the pericardial patch closure results in persistent VSR. Such patients are likely to respond favourably to percutaneous closure as the longer median time between MI and device implantation allows for better tissue strength around the defect to support the device.8–10
Factors Predicting Poor Outcomes
Procedures performed early within 14 days from the MI have higher rates of mortality, despite achieving procedural success. The purported mechanism is the lack of support from the friable tissue at the edges of the septal rupture, which results in incomplete sealing and subsequent residual shunting.9,11,12 There is also potential extension of the defect after the initial closure. However, delaying intervention in these patients results in higher mortality because of rapid progression to irreversible multi-organ failure. Improved survival shown in delayed intervention likely demonstrates survivorship bias.1 Hence, in patients presenting with cardiogenic shock, the decision to undertake earlier interventions must be taken on a case-by-case basis after comprehensive discussion by the heart team.
The presence of cardiogenic shock is a strong predictor of mortality irrespective of closure.8 Larger defects have greater left-to-right shunting and are prone to the development of cardiogenic shock.13 Unfortunately, these patients generally do not respond to medical stabilisation and will require urgent surgery. Thiele et al. showed that, among patients in whom procedural success was achieved, those with cardiogenic shock had a mortality of 86% compared with 36% mortality in non-shock patients. This study was performed prospectively with closure attempted if feasible, regardless of haemodynamic status, negating selection bias.14
Another important factor is the anatomy of the rupture, which plays a crucial role in determining not only the success of closure but also the technical difficulty of crossing the defect. Simple defects are through-and-through defects, while complex defects are serpiginous tracts with a greater distance from start to end.15 Inferior MI is generally associated with more complex anatomy near the base, which is less amenable to closure.16 Assessment of the morphology of the defect is difficult. Balloon sizing of the VSR is likely to underestimate the size and does not provide information on the complexity.10 Some operators have recommended oversizing the waist of the closure device to compensate for further enlargement of the defect post-procedure.7 One theoretical concern with balloon sizing is the risk of disruption or extension of the defect.8
Device Sizing and Selection
The VSR is most often sized with TOE in 2D or in 3D if available. Prior to the procedure, a CT may be performed if the patient is stable enough, which may aid in further sizing and understanding of the anatomy of the defect. Balloon sizing is another method advocated to confirm the estimated size of rupture.12 However, there is a potential risk of extension of the defect with balloon sizing.
Operators have used both atrial septal defect (ASD) and muscular VSD devices off-label for VSR closure. One of the commonest devices used is the Amplatzer muscular ventricular occluder (Abbott). Atrial septal occluders – particularly the earlier generation – are less ideal because of the shorter waist, which leads to device deformation and persistent shunting.14 The fabric composed of nitinol mesh and polyester was created to withstand transatrial pressures, which are considerably lower leading to increased permeability in VSR closure.
The Amplatzer post-infarction muscular VSD closure device (Abbott) is specifically manufactured for VSR closure. The main engineering difference is the wider and longer waist to accommodate damaged septal tissue, which tends to extend for long distances and can further expand due to ongoing tissue loss.
Antiplatelet and Antibiotic Therapy
Patients require antiplatelet therapy to reduce thromboembolic complications before the neoepithelialisation of intracardiac devices.17 Most recommendations for the duration and intensity of therapy are extrapolated from practices from congenital device closures. Most centres recommend 6 months of dual antiplatelet therapy with aspirin and a P2Y12 inhibitor, followed by aspirin monotherapy; longer duration may be given as per the requirements of treatment of acute coronary syndrome.1
Intravenous cefazolin is usually given 30 minutes to 1 hour before skin puncture. For those with penicillin allergy, vancomycin is administered 2 hours prior instead.18 Patients should also receive appropriate antibiotic prophylaxis prior to invasive procedures performed within 6 months of implantation.19 Longer term antibiotic prophylaxis may be required if there are residual leaks.
Techniques
Approaches
There are several approaches described for the percutaneous closure of VSR. Firstly, a primarily venous approach with the defect crossed from the RV. This approach can be considered if the defect is simple. However, crossing of the defect from the RV may be more challenging because of the trabeculation of the RV and the serpiginous shape of the rupture.13 Furthermore, the support provided by a solitary venous approach may be lacking, with difficulty in the advancement of the larger bore delivery sheaths. Secondly, dual venous access forming a venous–venous rail via a transseptal puncture has been used. In the venous–venous approach, a catheter is placed in the right atrium and a transeptal puncture is performed to allow access into the left atrium and ventricle. The catheter is then advanced into the LV positioned near the defect to allow the wire to cross into the pulmonary artery. Again, through the internal jugular sheath, the wire is snared and exteriorised. Thirdly, there is the arterial and venous approach to form an AV rail. The authors favour the third approach, which allows for much better support and the avoidance of a transseptal puncture. The subsequent discussion will focus on this later approach.
Vascular Access
Vascular access is recommended to be performed under ultrasound guidance. We recommend femoral venous access, and this may be preclosed with a single ProGlide (Abbott) device to allow for better haemostasis after the procedure. A larger bore sheath (usually 14 Fr) can be used to allow for larger delivery sheaths to be inserted. IJ access may be used, but its location may be more cumbersome to the operator with potentially increased radiation exposure. For the arterial access, a 6 Fr long sheath is recommended, which can help provide additional support.
Placement of Snare
A goose neck snare is placed in the pulmonary artery. This can be done by advancing a Swan Ganz (Edwards Lifescience) into the pulmonary artery and exchanging catheters over a long exchange 0.025 inch wire. Alternatively, diagnostic catheter (pigtail; Gensini, Balton) can be used to access the pulmonary artery and the snaring catheter exchanged over a long exchange 0.035/0.038 inch wire. The use of a larger goose neck snare may allow for easier snaring of the crossing wire.
Crossing of the Ventricular Septal Rupture
The left anterior oblique cranial project is generally used for crossing. If the patient is not too unstable, the performing of a left ventriculogram in this projection will allow for better visualisation of the location of the defect. The common catheters used are a JR4 or Internal Mammary catheter (Merit Medical), which allows for better positioning and manipulation to face the defect within the LV. A Terumo angled Glide wire (Terumo) is commonly used for the crossing.
Creation of the Arteriovenous Rail
Snaring of the wire is then performed and the wire exteriorised to create a complete AV loop. Snaring is best performed in the pulmonary artery rather than the RV as there are fewer issues with chordal entanglement and easier manipulation within the smaller space of the pulmonary artery.
Delivery of the Device
A long sheath is placed over the wire across the rupture. The sheath is placed from the RV into the LV. The device is then deployed through the sheath with the left disc expanding in the LV. The sheath is then withdrawn through the defect into the RV allowing the right disc to deploy on the RV side while simultaneously pulling on the left disc to complete opposition on the LV wall. The seal and stability can be assessed angiographically with a ventriculogram and/or TOE. If the position is unsatisfactory, the devices can be repositioned.
Conclusion
Advancements in percutaneous techniques and devices have provided a potential alternative to high-risk surgery in the management of VSR. However, this is a complex procedure in an often-fragile patient subset, emphasising the need for careful patient selection, multidisciplinary team management and building of strong technical expertise. 
Clinical Perspective
- Ventricular septal rupture portends significant mortality and morbidity.
- Percutaneous closure of post-infarction ventricular septal rupture may be a reasonable alternative in select patients.
- Anatomical factors such as site, size and morphology play an important role when considering percutaneous options.
- Systematic planning and a meticulous technical approach are crucial for performing this complex procedure.