Transcatheter aortic valve implantation (TAVI) is currently an established therapy for elderly patients with symptomatic severe aortic valve stenosis (AS) across all surgical risk categories. Rheumatic AS has been excluded from major TAVI trials primarily due to the difference in underlying pathology, which is postinflammatory commissural fusion and fibrinous thickening of the valve without calcification. The absence of calcification and the inability to adequately anchor the transcatheter aortic valve (TAV) poses an additional risk of valve embolisation, paravalvular regurgitation and conduction disturbances in patients with rheumatic AS. Here, we report a case of TAVI using a novel top-down deployment supra-annular leaflets valve in a patient with rheumatic AS and mechanical mitral valve prosthesis to illustrate the important technical considerations during TAVI when treating rheumatic AS.
Case Presentation
A 65-year-old man with chronic rheumatic heart disease and mechanical mitral valve replacement surgery performed 20 years ago was referred for TAVI in view of symptomatic, severe AS. Preprocedural CT showed an aortic annulus perimeter of 72 mm, left coronary artery height of 12 mm and right coronary artery height of 15 mm. The baseline coronary angiogram was normal. The patient was deemed high surgical risk for surgical aortic valve replacement in a heart team meeting and was therefore scheduled for TAVI with an ACURATE neo2 M-size TAV (Boston Scientific).
The ACURATE neo2 TAV delivery system was introduced over a Safari extra-small preshaped stiff guidewire (Boston Scientific). The flush port was maintained facing the 6 o’clock position during insertion. After crossing the aortic arch and reaching the aortic annulus level, the orientation of the commissural posts of the ACURATE neo2 TAV relative to the native aortic cusps was first assessed at the conventional three-cusp coplanar view, as described previously.1,2 There were three potential scenarios:
- Scenario 1: A ‘slit-like’ appearance of one of the commissural posts could be isolated and identified on the right side of the screen on the three-cusp view.
- Scenario 2: A ‘slit-like’ appearance of one of the commissural posts could be identified on the left side of the screen on the three-cusp view (Figure 1A).
- Scenario 3: No single commissural post could be identified on the left or right side of the screen on the three-cusp view.
For Scenario 1, gentle clockwise rotation of the delivery system would result in neo-commissural alignment, which appears as a single ‘slit-like’ appearance of one of the commissural posts on the right side of the screen on the cusp-overlap view (i.e. overlap of left and right coronary cusps, as predicted on preprocedural CT).3,4 For Scenario 2, gentle counterclockwise rotation of the delivery system would result in neo-commissural alignment, which could be confirmed on the cusp-overlap view (Figure 1B). For Scenario 3, the commissural posts are either perfectly aligned or completely misaligned. Again, this can be confirmed on the cusp-overlap view. No rotation of the delivery system would be required if the commissural posts are perfectly aligned. Either clockwise or counterclockwise rotation would be required if the commissural posts are completely misaligned. The major advantage of this approach is that the degree of rotation required would be kept to a minimum to optimise neo-commissural alignment.
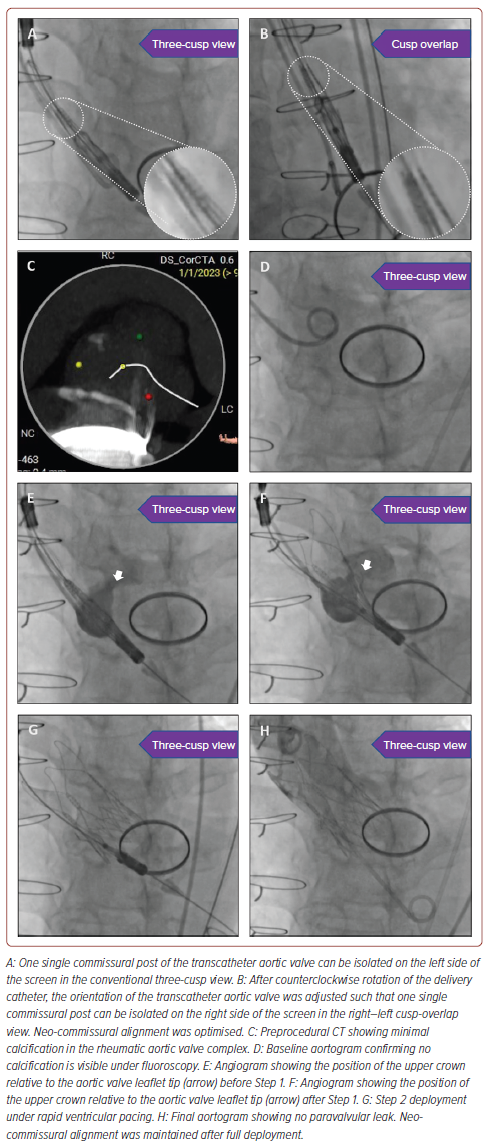
Deployment of the ACURATE neo2 TAV was then performed in the conventional three-cusp coplanar view. The rheumatic aortic valve complex had only minimal calcification, which was identified on both preprocedural CT (Figure 1C) and the baseline aortogram (Figure 1D), for TAV anchoring. During Step 1 deployment, repeated small puffs of contrast were required to ensure the upper crown deployment was right above the aortic valve leaflet tip (Figures 1E and 1F). Slow rotation of Knob 1 was also performed such that the position of the TAV could be immediately adjusted, if necessary. During Step 2 deployment, ultrarapid rotation of Knob 2 was performed under rapid ventricular pacing to ensure a stable TAV position relative to the native aortic valve during lower crown release (Figure 1G).
The final aortogram showed there was no paravalvular leak (Figure 1H). There was no interaction of the TAV with the existing mechanical mitral prosthesis. Neo-commissural alignment was maintained after full deployment. There was no conduction disturbance after the procedure. The patient was discharged the next day after the procedure. The 1-month follow-up transthoracic echocardiogram showed normal left ventricular ejection fraction, a transaortic mean gradient of 6 mmHg and trivial paravalvular leakage.
Discussion
The use of both balloon-expandable valves and self-expandable valves during TAVI to treat rheumatic AS has been reported in the literature, with comparable outcomes to those seen for degenerative AS.5 Yet, no studies have directly compared outcomes with balloon-expandable valves versus self-expandable valves in patients with rheumatic heart disease. This is the first report of the technical aspects of TAVI for rheumatic AS using the ACURATE neo2 TAV. There are several factors to consider when using this TAV in this special group of patients. First, during the initial deployment, the relative position of the upper crown of the TAV and native valve leaflet tip remains critical for TAV anchoring. A repeat angiogram is recommended to confirm leaflet tip position if there is no visible calcification for reference. Second, rapid ventricular pacing is essential during Step 2 deployment to ensure stable release of the lower crown in a calcium-free aortic valve complex. Third, considering the lifetime management of younger patients undergoing TAVI, neo-commissural alignment should always be optimised for future coronary re-access and the possibility of TAV-in-TAV. Recently, the NeoAlign study assessed neo-commissural alignment of the ACURATE neo2 in 170 consecutive patients.2 The authors assessed the orientation of the commissural posts and wings of the neo2 in the right–left cusp overlap view and three-cusp view and torqued the catheter to achieve the optimal orientation before deployment of the TAV. Post-deployment fluoroscopic assessment showed that successful alignment was achieved in 97% of patients, with the three easily identifiable commissural posts and the superior torque ability of the TAV delivery system contributing to the favourable result. Finally, the choice of TAV with a relatively lower rate of permanent pacemaker implantation should also be taken into consideration when treating younger patients with longer life expectancy. The recently published ACURATE neo2 PMCF study evaluated the clinical outcomes and valve performance at 1 year in patients with severe AS treated with the ACURATE neo2 TAV.6 In that study, the newly implanted permanent pacemaker rate at 30 days and 1 year was 6.1% and 7.8%, respectively.
Conclusion
This case report demonstrates the feasibility of TAVI in treating rheumatic AS using the top-down deployment supra-annular leaflets ACURATE neo2 TAV. Multiple technical considerations must be taken into account during TAVI when treating this subset of patients.
Clinical Perspective
- Chronic rheumatic heart disease is a common cause of aortic valve stenosis in the Asia-Pacific region.
- Transcatheter aortic valve implantation (TAVI) is an established therapy for elderly patients with symptomatic severe aortic valve stenosis across all surgical risk categories.
- Lack of calcification in the rheumatic aortic valve complex poses technical challenges for TAVI.
- Certain technical tips and tricks are essential when using a top-down deployment supra-annular leaflets TAVI to treat rheumatic aortic valve stenosis.










