With advances in devices and techniques of percutaneous coronary intervention (PCI), interventional cardiologists are managing patients with coronary artery disease (CAD) of increasing complexity. CAD prevalence is rapidly rising with an ageing population, and recognition that treatment goals may be different in this population is important. In the US, among adults 75–84 years of age, the prevalence of CAD is expected to double from approximately two million in 2010 to more than 4 million in the next 30 years. Fifty per cent of MIs are projected to occur in patients aged ≥65 years, with an 80% mortality rate in patients aged ≥70 years.1,2
Studies have demonstrated that revascularisation in the form of coronary artery bypass graft (CABG) confers survival benefit over medical treatment in patients with left main disease, triple-vessel disease (TVD) and ischaemic cardiomyopathy.3–13 However, for patients with prohibitive operative risk or those with a personal preference regarding treatment (such as refusing CABG), PCI may provide a reasonable alternative.
Procedural haemodynamic stress often poses a significant challenge and is poorly tolerated in patients with reduced myocardial reserve, such as those with advanced heart failure or extreme frailty. Therefore, the risks of cardiac arrest and death during PCI are significantly higher in these patients.
Emergence of new mechanical circulatory support (MCS) devices has made PCI a more viable option for these high-risk patients. In this article, we review the existing evidence on the use of MCS in non-emergency complex and high-risk PCI, the optimal timing of initiating MCS and barriers to MCS use.
Defining High-risk Percutaneous Coronary Intervention
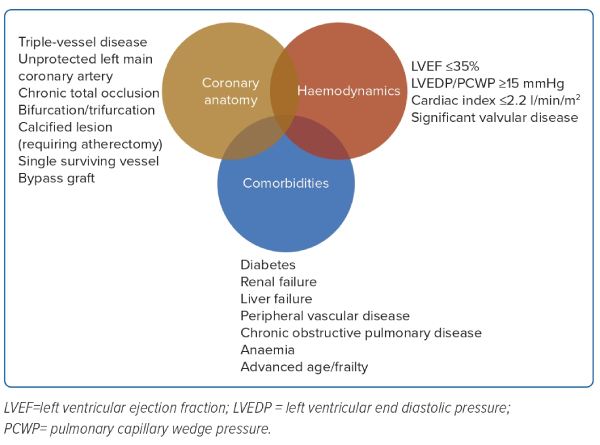
Complex, high-risk and indicated PCI (CHIP) is loosely and heterogeneously defined in the literature. There is growing opinion that this definition should centre around three parameters: haemodynamic status; coronary anatomy; and clinical comorbidities.14–16 These factors are summarised in Figure 1.
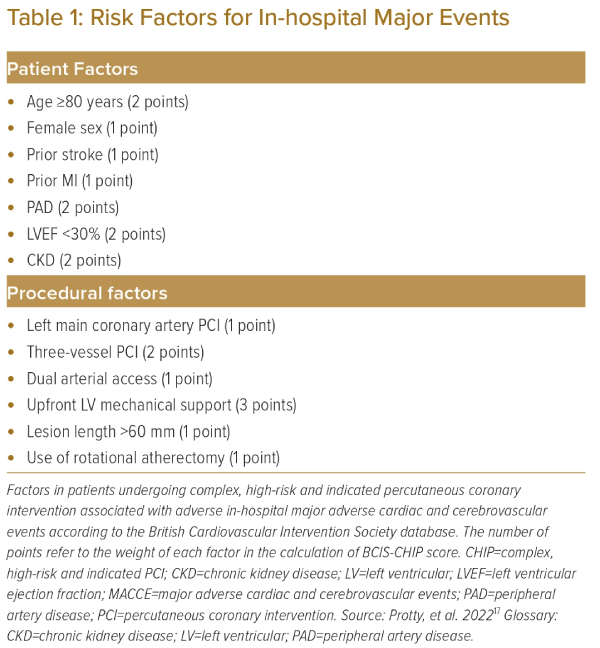
More recently, the British Cardiovascular Intervention Society (BCIS) database was analysed using a multiple logistic regression model to identify 13 variables associated with major in-hospital adverse events with the objective of constructing a CHIP score.17 The study identified seven patient factors and six procedural factors associated with in-hospital adverse events (Table 1). Each factor was assigned points depending on the observed odds ratio for adverse events compared to baseline. The study observed there was an exponential increase in in-hospital adverse events with a rise in the CHIP score.
This CHIP score was further validated in a PCI database including 20,779 patients at Mount Sinai Hospital in New York, US.18 Analysis showed that, among patients with a CHIP score of ≥5, the risk of major adverse cardiac and cerebrovascular event (MACCE), a composite of all-cause death, MI or stroke at 1 year, was up to 12%.
We believe that a universally agreed definition of CHIP is of increasing importance in the field of interventional cardiology. The use of a standardised definition could enhance the generalisability of future CHIP research and help provide an objective assessment of procedural performance in the catheterisation laboratory as the expected mortality is different from that in ordinary elective cases.
Trends in High-risk Percutaneous Coronary Intervention
With an ageing population and increasing complexity in both medical comorbidities and coronary anatomy, the volume of high-risk PCI is progressively rising. According to a cohort study in the Veterans Affairs Administration in the US, the proportion of high-risk PCI has significantly increased between 2008 and 2018.19 In an Australian PCI cohort of over 40,000 procedures from 2005 to 2018, a significant increase in procedural complexity as measured by CathPCI National Cardiovascular Data Registry mortality risk scores has been observed.20 In the UK, there was also a significant increase in PCI procedural complexity as measured by the newly proposed CHIP score between 2006 and 2016, according to a BCIS database of over 300,000 patients.17
As the volume of high-risk PCI increased, there has also been a rise in the use of mechanical devices to support these patients. The intra-aortic balloon pump (IABP) remains the most popular MCS for this purpose, although there has also been a rapid increase in the use of micro-axial pump MCS (e.g. the Impella [Abiomed]).
A database that includes around 20% of all acute care hospitalisations in the US shows the use of MCS has increased from 2.5% of all PCI procedures to 3.5% between 2004 and 2016. Approximately 90% of patients undergoing PCI with MCS during this period received an IABP, although the use of the Impella steadily increased from <1% in 2,008 to >30% in 2016 since the device received approval and funding.21
Another database of all-payer hospital inpatient stays in the US showed a 27-fold increase in the number of Impella or TandemHeart (CardiacAssist) assisted PCI procedures between 2008 and 2018.22
In Europe, according to a multicentre observational registry covering 17 centres in Italy, the use of the Impella for cardiogenic shock and high-risk PCI has increased by an average annual rate of nearly 40% between 2004 and 2018.23
While the Impella has been introduced in different parts of Asia over the past decade, its use in high-risk PCI has been significantly lower than in North America and Europe, in part because of the cost of the device.24
Mechanical Circulatory Support Options and Evidence
Different percutaneous mechanical circulatory devices have been used to support patients vulnerable to haemodynamic compromise during high-risk PCI. During interventions undertaken in complex coronary anatomy, such as prolonged balloon inflation, kissing balloon inflation, single remaining patent vessel intervention, left main coronary artery intervention, retrograde chronic total occlusion intervention or atherectomy, there is an increased risk of significant myocardial ischaemia. Such ischaemia might be poorly tolerated by patients with underlying conditions such as reduced left ventricular (LV) systolic function or significant frailty.
Mechanical circulatory support during high-risk PCI might be helpful in maintaining a stable haemodynamic condition for these patients and reduce major adverse effects associated with the procedure.
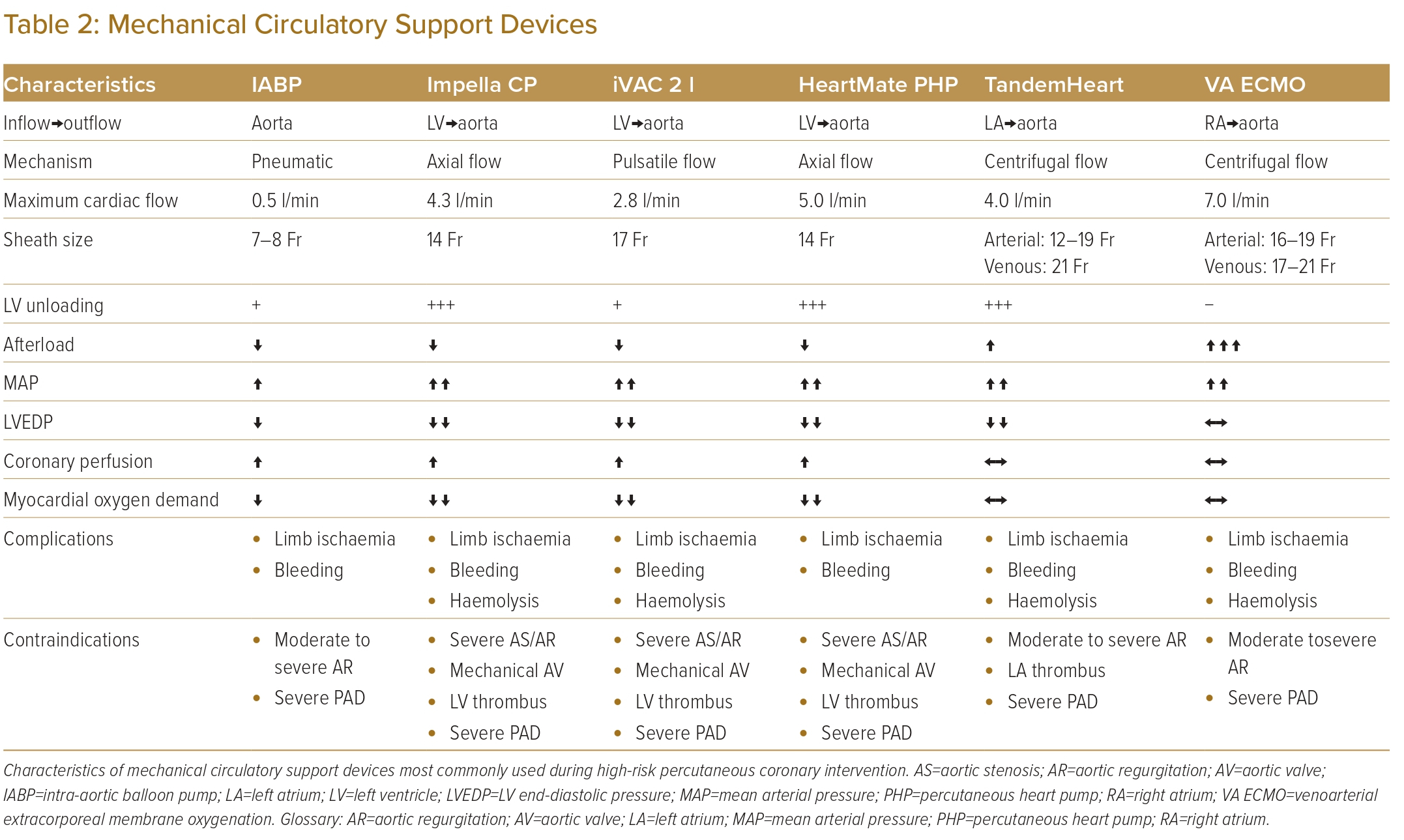
Table 2 summarises the characteristics of support devices most commonly used during high-risk PCI.
Intra-aortic Balloon Pump Counterpulsation
The intra-aortic balloon pump was first introduced in 1968 as a cardiac support device for the management of cardiogenic shock following MI.25 It is also the first available and most widely used haemodynamic support device in high-risk PCI.
During the procedure, the balloon is inserted most commonly via the common femoral artery through a 7–8 Fr catheter. Insertion via the subclavian and axillary arteries have also been reported. The balloon is placed in the descending aorta distal to the origin of left subclavian artery and proximal to the renal arteries.26,27
During diastole, the balloon inflates to augment coronary blood flow. During systole, the balloon rapidly deflates to create a vacuum effect to reduce afterload and decrease myocardial oxygen demand.28,29 Its action could increase cardiac output by up to 0.5 l/min, the extent to which depends on LV contractility – the poorer the LV systolic function, the less the haemodynamic improvement.30 Moreover, the IABP has not been shown to significantly improve distal coronary blood flow in the presence of critical coronary stenosis.29,31
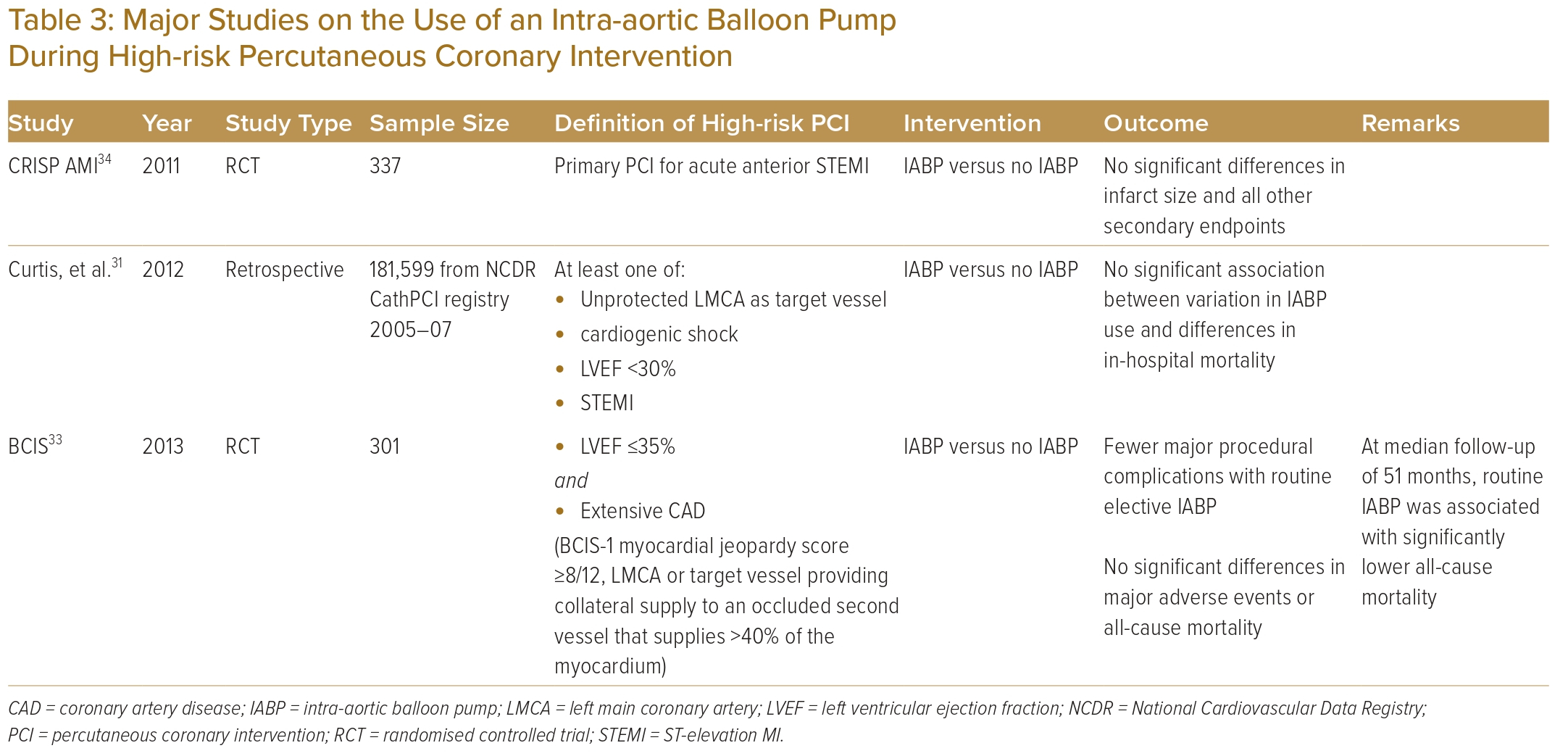
The role of the IABP in high-risk PCI was investigated using data from the National Cardiovascular Data Registry: CathPCI Registry, which defined PCI as high risk in patients with unprotected left main artery as the target vessel, cardiogenic shock, severely depressed LV function or ST-elevation MI (STEMI). The study included 181,599 high-risk PCI cases across 681 centres in the US from 2005 to 2007.32 Of all the high-risk PCIs performed, 10.5% were supported by an IABP. The study failed to demonstrate any significant association between variation in IABP use and differences in in-hospital mortality.
BCIS-1 was the first randomised controlled trial studying the outcome of routine use of an IABP before high-risk PCI. The trial was conducted at 17 centres in the UK and included 301 patients with LV ejection fraction (LVEF) ≤30% and extensive coronary artery disease. While fewer major procedural complications occurred in patients with routine elective IABP insertion compared with those with no planned IABP, there were no significant differences in major adverse events or all-cause mortality. However, in an extended study with a median follow-up of 51 months, routine IABP use during PCI was associated with significantly lower all-cause mortality as compared with PCI alone.33
CRISP AMI was a randomised controlled trial that investigated the benefit of routine IABP implantation before primary PCI in the setting of acute anterior STEMI without shock.34 The study randomised 337 patients at 30 sites in nine countries to either an IABP plus PCI or PCI alone. There were no significant differences between the two groups with regards to infarct size and all other secondary endpoints.
Table 3 summarises the evidence on IABP use in high-risk PCI.
Impella
The Impella is a continuous centrifugal axial pump that can support the left and right ventricles.
The left ventricle pumps are available at peak flow rates of up to 2.5 l/min (Impella 2.5), 4.3 l/min (Impella CP), 5.0 l/min (Impella 5.0) and 6.0 l/min (Impella 5.5).
Insertion sites include common femoral, axillary, subclavian arteries and trans-caval, depending on the catheter size required by individual model. The left ventricle support devices are positioned across the aortic valve. They unload the left ventricle by drawing blood from this chamber and ejecting it into the ascending aorta. Impella-assisted LV unloading increases mean arterial pressure, reduces LV end-diastolic pressure and, in turn, improves coronary perfusion pressure. During high-risk PCI, the Impella was found to reduce LV end-diastolic wall stress, increase LV diastolic compliance and reduce coronary microvascular resistance.35,36
The Impella RP, which has a different configuration, provides support to the right ventricle; this is beyond the scope of this paper.
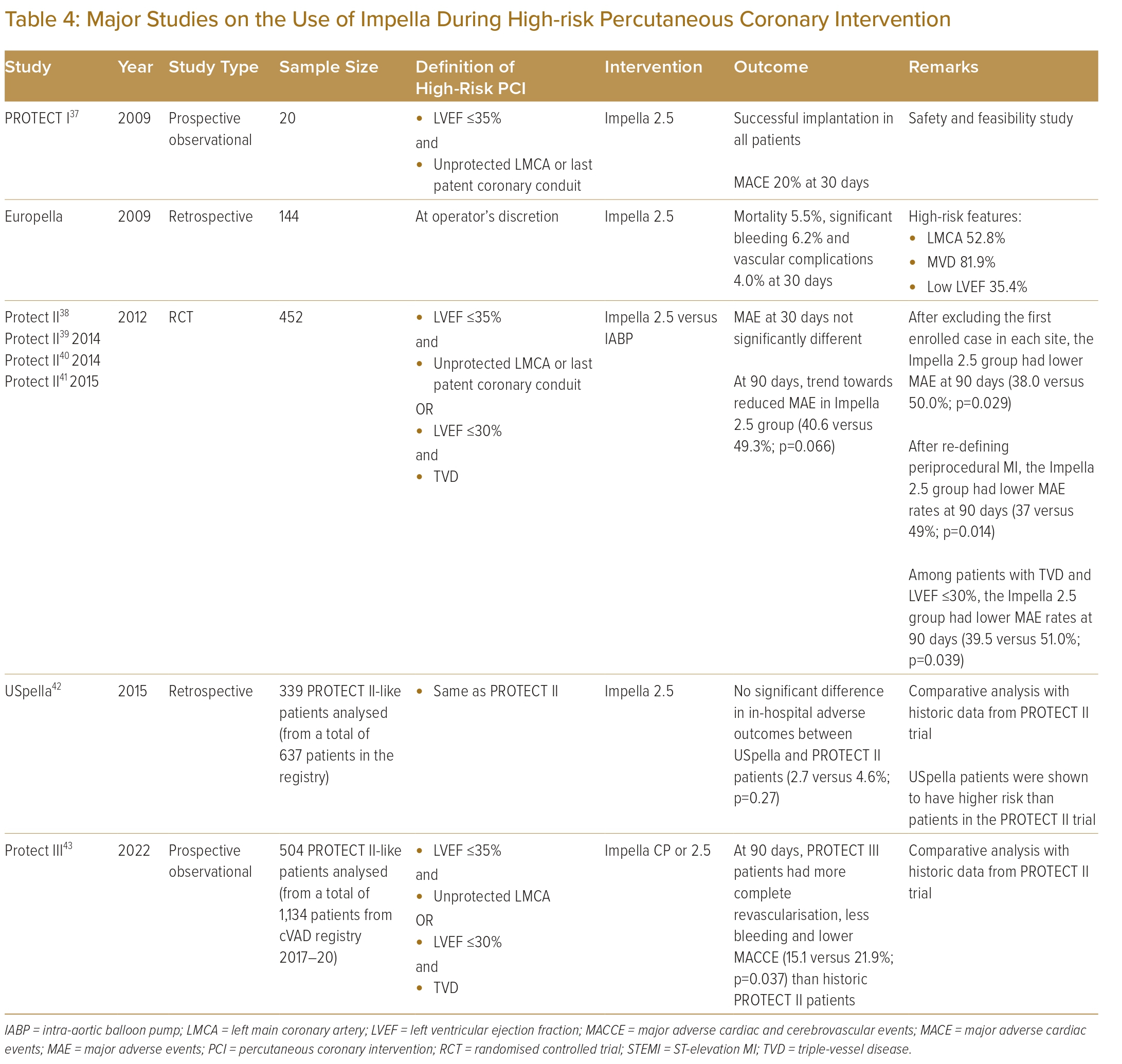
The PROTECT I trial was a prospective multicentre study evaluating the safety and feasibility of using the Impella 2.5 in patients undergoing high-risk PCI.37 The trial enrolled 20 patients from seven centres with LVEF ≤35% who underwent PCI to either an unprotected left main coronary artery or a single remaining vessel. Two patients had a periprocedural MI and two died within 30 days. None experienced haemodynamic compromise during PCI.
The PROTECT II trial was a prospective, multicentre randomised controlled trial (RCT) designed to compare the use of the IABP versus the Impella 2.5 in symptomatic patients undergoing high-risk PCI.38 To date, the PROTECT II trial remains the first and only RCT on the use of MCS in high-risk PCI. Patients were eligible for inclusion if they had LVEF ≤35% requiring PCI to an unprotected left main coronary artery disease or single remaining vessel, or if they had an LVEF ≤30% and three-vessel disease. The study enrolled 452 patients across 112 international sites, with four dropouts before undergoing PCI. The study was discontinued for futility but did report haemodynamic support provided by the Impella 2.5, which was superior in comparison with the IABP. The study failed to demonstrate a statistically significant difference in 30-day incidence of major adverse events between the two groups. At 90 days, there was a trend towards lower rates of major adverse events in patients supported by the Impella 2.5, but the difference was not statistically significant (p=0.066).
To take account of the learning curve in the use of MCS, a subset of patients was re-analysed after the first case enrolled at each site was excluded.39 In this subset, patients supported by the Impella 2.5 had a significantly lower rate of major adverse events at 90 days (38.0 versus 50.0%; p=0.029). In a subsequent analysis, the definition of periprocedural MI was changed from ≥3 times the upper limit of normal for cardiac biomarker elevation to a more stringent version using new Q-waves or a creatine kinase-MB elevation >8 times normal. With this revised definition of periprocedural MI, the Impella 2.5 group was then found to have a statistically significant lower 90-day major adverse event rate than the IABP group (37 versus 49%; p=0.014).40 In a sub-analysis of patients with LVEF ≤30% and three-vessel disease, those supported by the Impella 2.5 had a significantly lower incidence of major adverse events at 90 days than those supported by the IABP (39.5 versus 51.0%; p=0.039).41
The USpella registry was an observational multicentre registry of Impella use across 47 centres in the US and two centres in Canada.42 Of the 637 patients who underwent high-risk PCI with the Impella 2.5, 339 patients who met PROTECT II eligibility criteria were included for analysis. These 339 patients were then compared with the 216 patients treated with an Impella in the PROTECT II trial. Although patients from the USpella registry were older and had more comorbidities, clinical outcomes were not significantly different, and there was a trend towards lower in-hospital mortality for the registry patients. Based on data from the PROTECT trials and the USpella registry, the Impella 2.5 received pre-market approval from the Food and Drug Administration for use in elective and urgent high-risk PCI in 2015.
In 2016, the USpella registry was replaced by the cVAD registry. The cVAD registry is an ongoing, prospective international database that continues to assess the use of Impella to support high-risk PCI, cardiogenic shock and decompensated heart failure.43 It captures both in-hospital and 1-year postprocedural outcomes.
Based on the cVAD registry, PROTECT III was designed as a single-arm study to evaluate the new Impella CP, a more powerful pump than the Impella 2.5, to support complex PCI in patients with depressed LV systolic function in the setting of contemporary practice in the use of mechanical support.44 The study enrolled 1,143 patients undergoing elective non-emergent PCI supported by the Impella 2.5 or Impella CP at 45 sites. Of these patients, 504 were met the eligibility criteria for the PROTECT II trial and were included for comparative analysis with PROTECT II patients. Of all PROTECT III patients, 68.1% received an Impella CP with the rest supported by an Impella 2.5. Compared with patients in the PROTECT II trial, PROTECT III patients were older with more comorbidities and more complex coronary anatomy and they received more extensive and complete revascularisation including the use of rotational atherectomy. The study demonstrated that patients in PROTECT III study had significantly less in-hospital bleeding requiring transfusion, procedural hypotension or ventricular arrhythmia and were less likely to need cardiopulmonary resuscitation. At 90 days, the rate of major adverse events was significantly lower in PROTECT III patients (15.1 versus 21.9%; p=0.037).
Moving forward, the PROTECT IV study is an ongoing, multicentre RCT investigating high-risk patients with complex coronary artery disease and impaired LV systolic function undergoing PCI with or without Impella support (NCT04763200). The study aims to enrol 1,252 patients from over 100 centres across Europe and the US with follow-up for up to 3 years. The study is anticipated to be completed by March 2026.
Existing evidence regarding use of the Impella in high-risk PCI is summarised in Table 4.
The Impella ECP, a smaller version of its predecessor, requires 9 Fr arterial access and provides a peak flow rate of >3.5 l/min. Its use in high-risk PCI will be evaluated in the ongoing prospective multicentre single-arm Impella ECP Study (NCT05334784). It remains to be seen whether access using a smaller cannula of 9 Fr might significantly reduce the risk of vascular complications associated with the use of existing Impella devices.
TandemHeart
The TandemHeart is a centrifugal pump that supports the left ventricle by providing a left atrium to femoral artery bypass. During implantation, an arterial cannula is positioned in the iliofemoral arterial system while a venous cannula is inserted via the femoral vein and placed in the left atrium via transseptal puncture. Compared with the Impella and the IABP, implantation of the TandemHeart is technically more demanding as it requires large-bore access (21 Fr venous cannula and 17 Fr arterial cannula) and transseptal puncture.
Early experience of the use of the TandemHeart in high-risk PCI was reported in small registries in which the TandemHeart appeared to provide adequate support and enhance procedural safety in critically ill patients.45–48
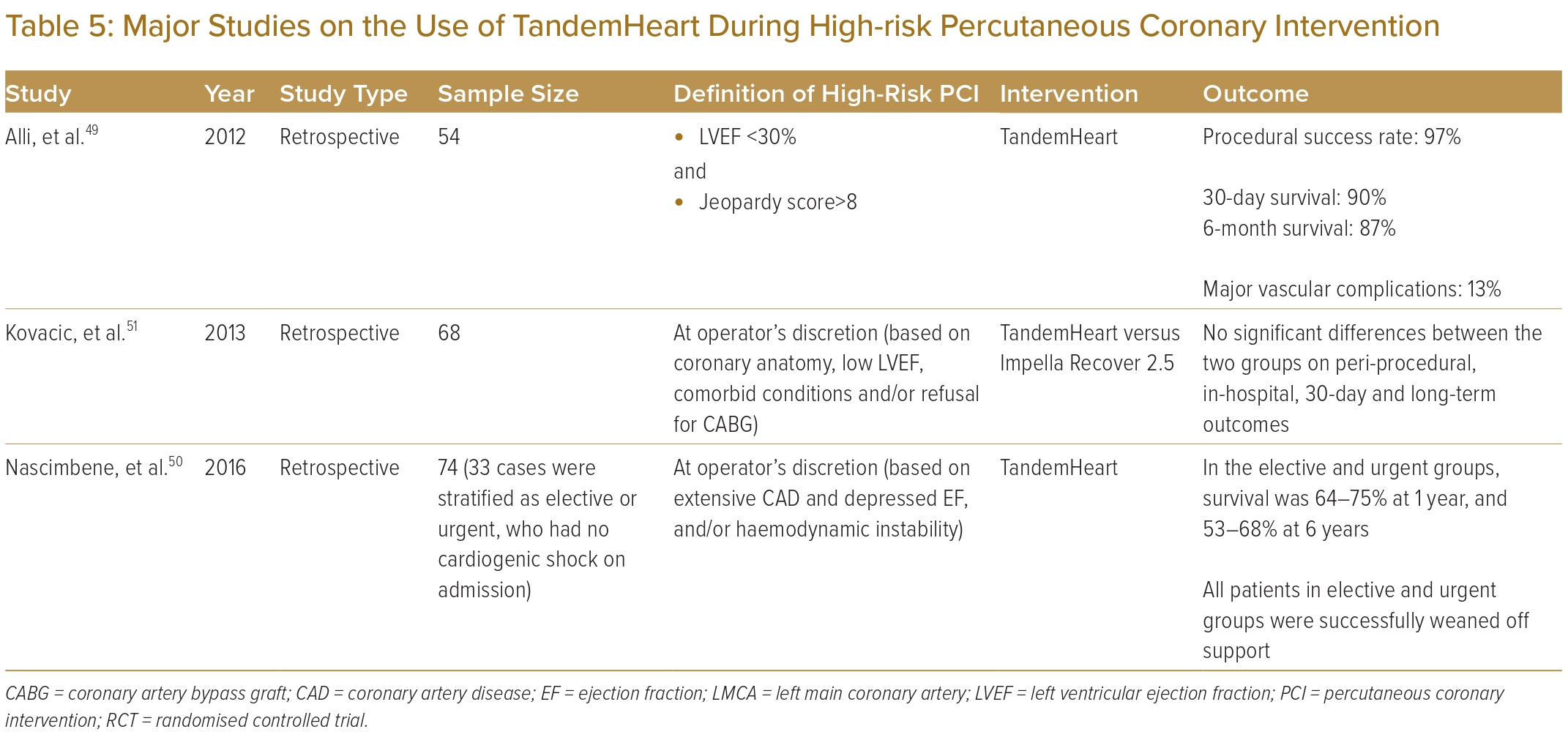
The Mayo Clinic performed a retrospective analysis of 54 patients who were supported by a TandemHeart during high-risk PCI.49 These patients had a mean age of 72±1.7 years, a median LVEF of 20% and a median SYNTAX score of 33. Half of them had a history of prior CABG. During the procedure, cardiac output increased from 4.7 to 5.7 l/min with a significant drop in bilateral heart pressures. PCI was successful in 97% of patients, with a 6-month survival rate of 87%. Thirteen per cent of cases had major vascular complications.
Another group, from Texas in the US, evaluated 74 PCIs supported by a TandemHeart from 2005 to 2011.50 Among patients without cardiogenic shock who were not eligible for surgery because of coronary anatomy or comorbidities, survival rates were 88–94% at 30 days, and 64–75% at 1 year.
A single-centre retrospective study compared the use of the TandemHeart with the Impella Recover 2.5 during high-risk PCI.51 Of the 68 patients evaluated, 32 received the TandemHeart while the others were supported by the Impella Recover 2.5. One patient experienced left atrial perforation during TandemHeart implantation. Overall, the study reported no significant differences in terms of in-hospital, 30-day and long-term clinical outcomes.
To date, there have been no RCTs on the use of the TandemHeart in high-risk PCI.
Table 5 summarises available evidence on the use of the TandemHeart during high-risk PCI.
Veno-arterial Extracorporeal Membrane Oxygenation
Veno-arterial extracorporeal membrane oxygenation (VA-ECMO) is a cardiopulmonary support system that provides a continuous, non-pulsatile cardiac output. It was originally reported in 1979 as a system to support respiratory failure.52 It is the only MCS device that oxygenates blood by removing carbon dioxide from and adding oxygen to venous blood through an artificial membrane, bypassing the pulmonary circulation.
During implantation, an arterial cannula is inserted via the femoral artery and placed in the aorta, while a venous cannula is inserted via the femoral vein and positioned in the right atrium. In patients with reduced LV systolic function, the increased afterload following VA-ECMO support leads to LV distension, and increases in LV wall stress and myocardial oxygen demand.53,54 Therefore, an LV unloading strategy such as an IABP or Impella is often required to provide optimal cardiac output and as a venting strategy to avoid persistent aortic valve closure and LV thrombosis.
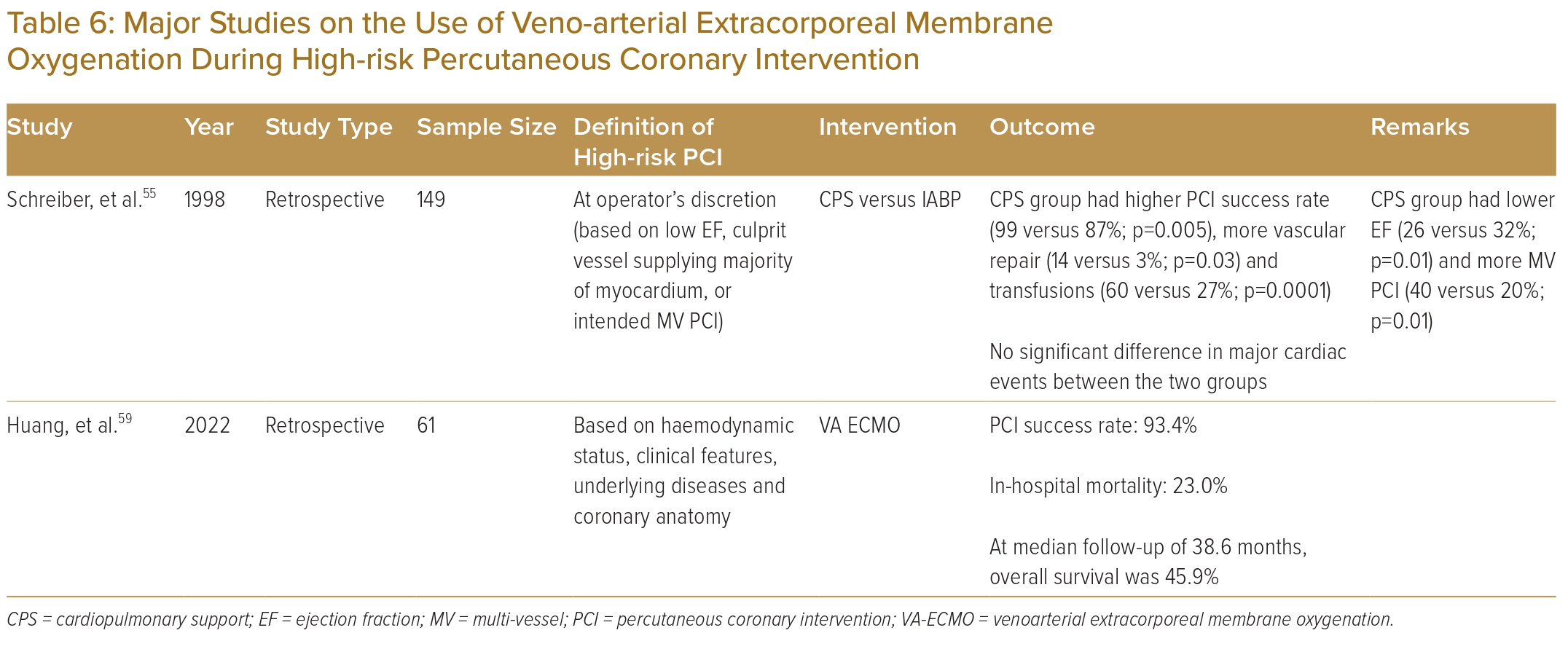
A retrospective analysis compared patients undergoing high-risk PCI supported by either the cardiopulmonary system or an IABP based on the physician’s preference.55 Patients supported by the cardiopulmonary system had higher risk profiles but tolerated longer balloon inflations and achieved higher procedural success rates compared with the IABP group. Since then, however, evidence regarding the use of VA-ECMO in non-cardiogenic shock patients undergoing high-risk PCI has been limited to case series.56–58 More recently, a group from China reported a retrospective cohort study of 61 patients who underwent high-risk PCI supported by VA-ECMO.59 The PCI success rate was 93.4%. At a median follow-up of 38.6 months, overall survival was 45.9%.
To date, there are no randomised controlled trial or meta-analysis data to support the use of VA-ECMO in high-risk PCI. Current evidence on the use of VA-ECMO in high-risk PCI is illustrated in Table 6.
The use of LAVA-ECMO (left atrial veno-arterial ECMO), first reported in 2018 for use in biventricular failure, has gained increasing attention in recent years.60 With transseptal placement of the venous cannula, both atria are drained simultaneously leading to decompression of the left ventricle without an additional MCS device such as an Impella. LAVA-ECMO has also been used to unload the left ventricle in patients with significant aortic regurgitation when conventional VA-ECMO, Impella and IABP use are all contraindicated.61,62 To date, data on the use of LAVA-ECMO in patients undergoing high-risk PCI have been limited.
Timing of Mechanical Circulatory Support
One of the biggest challenges in the decision-making process is the timing of MCS initiation with respect to the PCI procedure. The rationale supporting the prophylactic use of MCS in high-risk PCI is that it offers timely support in case of sudden haemodynamic collapse, safer puncture for large-bore access prior to therapeutic heparinisation and better tolerance to prolonged balloon inflation and atherectomy procedures. Advocates of standby support would argue that prophylactic use could incur unnecessary risks and monetary costs for patients who do not actually require that support during the procedure.
O’Neill, et al. performed a retrospective analysis of 1,028 patients from the cVAD and USpella registries who underwent PCI with either prophylactic or bailout Impella support.63 Of these patients, only 57 received an Impella as bailout support. In this study, the bailout group saw significantly increased mortality than patients who routinely received an Impella upfront (57.8 versus 4.4%; p<0.0001).
Tarantini, et al. reported a similar analysis based on 365 patients from the IMP-IT Registry.64 Consecutive patients treated with the Impella 2.5 or CP at 17 Italian centres were included. Of these patients, 174 received an Impella in the setting of high-risk PCI, with 57 Impella implantations being carried out during or after the PCI procedure mostly because of peri-procedural haemodynamic compromise. The study showed that, at 1 year, all-cause mortality (12 versus 3%; p=0.02) and severe bleeding (9% versus 1%; p=0.02) were significantly higher among patients who received an Impella during or after PCI.
The results from these two studies were unsurprising, given the substantial risk of delayed treatment for haemodynamic compromise and obtaining large-bore access under therapeutic heparinisation during bailout Impella implantation. However, these studies did not include patients who underwent high-risk PCI with standby Impella but ultimately did not require support. This lack of a control group makes it impossible to conclude whether any one strategy is superior. It should also be noted that these were retrospective studies.
Retrospective analysis of a multicentre registry data showed that, compared with standby support, prophylactic use of cardiopulmonary bypass to support high-risk PCI was associated with lower procedural mortality among patients with LVEF ≤20%. However, overall, patients receiving prophylactic cardiopulmonary support had significantly higher rates of vascular complications.65
A more reasonable approach would therefore be to select patients who would benefit from prophylactic support during high-risk PCI and differentiate them from those who could be safely managed with standby support. An ongoing single-centre registry has proposed a scoring algorithm based on different predictors of periprocedural haemodynamic compromise to guide patient selection for protected PCI in the setting of chronic total occlusion.66,67 Through this scoring algorithm, the researchers sought to identify patients who would be unlikely to need support and those for whom protected PCI should be considered. The final results, including on the predictive performance of this screening algorithm, are pending.
Future randomised controlled studies should aim to identify a list of high-risk features that characterise this highly selected group of patients who would benefit from prophylactic mechanical support. Patients who do not meet such criteria could ideally be spared from the potentially life-threatening bleeding complications arising from the use of percutaneous mechanical circulatory support devices and still have the PCI procedure completed safely.
Barriers to Mechanical Circulatory Support Use
The use of mechanical circulatory support in high-risk PCI has become increasingly popular in the US and Europe, although practice remains highly variable. In other parts of the world, however, the IABP remains the only option of support. The high cost of the Impella has been a major limiting factor.
In addition, RCT data suggest it provides no significant clinical benefit, although this is mitigated by subgroup and secondary analyses.
Cost and Availability
Using MCS in high-risk PCI is adds significant cost to the procedure. The device cost of the IABP is approximately US$800–1,000 while that of the Impella is in the region of US$23,000–28,000.68,69 The TandemHeart has a similar cost to the Impella.70 For ECMO, while the cost per circuit is US$2,500–14,000, the console can cost up to US$110,000.71
Other costs incurred can arise from monitoring, specialised personnel, training and management of complications such as those requiring transfusion, which could exceed the cost of the device itself. Such costs could deter the use of MCS, especially in the healthcare systems of developing countries.
A study in the US has shown that the adjusted costs of hospitalisation for patients undergoing PCI with MCS rose from US$47,000 to US$52,000 after the Impella was introduced.19 On the other hand, the proper use of MCS in suitable patients could potentially reduce adverse events during high-risk PCI and benefit those who would otherwise be declined revascularisation due to risks that are unacceptable to either patients or physicians. This would in turn reduce burdens on the healthcare system. Taking into account the short-term effectiveness and safety as well as the long-term risk of major adverse events, research has shown that the use of the Impella in high-risk PCI is cost-effective, even more so than the IABP.72,73
After its introduction more than five decades ago, the IABP remains widely available around the world. While the use of the Impella during high-risk PCI is increasingly common in the US and Europe, the situation is highly variable around the world. In Japan, Impella use remains restricted to patients with cardiogenic shock.74,75 In Hong Kong, Impella use in high-risk PCI has been subsidised by public funding for eligible patients since 2019 with over 30 cases performed under this programme from 2019 to 2020.69 In Australia, an application for public funding of Impella support in high-risk PCI and cardiogenic shock was turned down by the Medical Services Advisory Committee in 2019 due to uncertainty on comparative safety, effectiveness and financial estimates, thus limiting its use for most patients.76
Technical Requirements and Potential Complications
Implantation of MCS devices requires operators to obtain large-bore access, perform closure of vascular access after device removal and manage potential vascular complications. Implantation of the TandemHeart also involves transseptal puncture. These skills are technically demanding and are sufficiently acquired only after high-volume specialised training. Therefore, the use of MCS devices, with the exception of the IABP, are limited to highly specialised centres only.
Other common complications regarding use of MCS include thrombosis and, potentially, stroke, infection, particularly over the access site, and bleeding associated with systemic heparin use.
Device-specific complications are summarised in Table 2.
Insufficient Evidence
As discussed above, currently available evidence regarding use of MCS to support high-risk PCI is based predominantly on retrospective or observational data. There is a lack of large-scale, high-quality RCTs demonstrating clear clinical benefits in the use of any of the MCS devices during high-risk PCI. This can be explained by the heterogeneity of patients undergoing high-risk PCI, a lack of a universally accepted definition of high-risk PCI or risk calculators, the cost of MCS devices, variable practice and the availability of MCS devices in different centres.
Because of this, European Society of Cardiology guidelines did not make specific recommendations on the use of MCS during revascularisation while the American College of Cardiology made only a class IIb recommendation for the use of support device as an adjunct to PCI for selected high-risk patients to prevent haemodynamic compromise.77,78
Future Development
Ongoing Trials
Within currently available data on the use of mechanical circulatory support for high-risk PCI, there remain many evidence gaps regarding this rapidly evolving area of interventional cardiology. The key clinical gaps in existing evidence include:
- a universally accepted definition of CHIP;
- appropriate patient selection for protected PCI;
- optimal choice of support device for use during high-risk PCI; and
- optimal timing to initiate support and an algorithm on termination of support
Some RCTs are being carried out in an attempt to answer some of these questions.
The CHIP-BCIS3 study is a randomised controlled trial in the UK, which aims to determine whether the use of an LV unloading device is beneficial and cost-effective in patients with LVEF ≤35% (or ≤45% with severe mitral regurgitation) and extensive coronary artery disease (defined by BCIS jeopardy score ≥8) undergoing complex PCI (NCT05003817). Patients are randomly assigned to either PCI supported by an LV-unloading device or PCI without support. The primary outcome is a composite hierarchical outcome including mortality, stroke, MI or cardiovascular hospitalisation at a minimum follow-up of 12 months and up to 4 years. It aims to enrol 250 patients and to be completed by June 2026.
PROTECT IV is a prospective, multicentre randomised parallel-controlled trial, evaluating the use of the Impella CP (or 2.5) versus the IABP (or no support) during high-risk PCI in patients with complex coronary artery disease and reduced LV function (NCT04763200). The study is being carried out at over 100 sites across the US and four European countries. The investigators hypothesise that high-risk complex PCI supported by an Impella is associated with better stent optimisation and more complete revascularisation, which would in turn improve early and late outcomes. The primary endpoint is a composite of all-cause mortality, stroke, MI or cardiovascular hospitalisation at 3 years. The trial aimed to recruit 1,252 participants and is anticipated to be completed by March 2026.
New Devices
New mechanical support devices have also been investigated. The iVAC 2 l (PulseCath) is a new-generation membrane pump that is inserted percutaneously via a 21 Fr catheter, with the tip and valve positioned in the left ventricle and ascending aorta respectively. Driven by any standard IABP console, it generates a pulsatile blood flow of up to 2 l/min. The iVAC 2 l has had CE mark approval for LV circulatory support for up to 24 hours since February 2014.79
The feasibility and safety of iVAC 2 l support during high-risk PCI was demonstrated in a prospective single-centre, single-arm study of 14 patients.80 In this pilot study, the device was successfully implanted in 13 patients, resulting in significant increases in both mean arterial pressure and cardiac output relative to baseline values. Use of the iVAC 2 l during high-risk PCI was directly compared with the Impella 2.5 in a prospective non-randomised trial consisting of 40 patients.81 The first 20 patients were consecutively supported by an iVAC 2 l while the next 20 patients underwent high-risk PCI with an Impella 2.5. In comparison to the iVAC 2 l, the Impella 2.5 support generated significantly higher blood flow (2.07±0.09 l/min versus 1.25±0.05 l/min; p<0.001). Both the iVAC 2 l and the Impella 2.5 were associated with comparable increases in aortic pressure, although the increase by the iVAC 2 l was achieved later during the procedure.
The HeartMate percutaneous heart pump (Abbott) is another percutaneous support device under investigation. It is a catheter-based, axial flow support system with a collapsible pump inserted through a 14 Fr access site and deployed across the aortic valve.82 The system can deliver blood flow at up to 5 l/min. The SHIELD I trial, a prospective single-arm multicentre study, evaluated the use of the HeartMate system for patients with LVEF ≤35% undergoing high-risk PCI.83 The study enrolled 50 patients at seven centres in Europe and South America. At 30 days, no cardiac deaths, MIs or surgical interventions had taken place. Six complications occurred, including major bleeding, stroke, access site complications requiring intervention and new or worsening aortic regurgitation. CE mark approval for the short-term use of HeartMate percutaneous heart pump to support high-risk PCI was granted in July 2015 on the basis of data from the SHIELD I trial.
The SHIELD II trial was a prospective, randomised trial originally intended to compare the use of the HeartMate percutaneous heart pump versus the Impella during high-risk PCI. Enrolment began in 2015 but the study was terminated in 2017 because of several device malfunction events.84
Conclusion
Advances in PCI techniques and the emergence of new percutaneous mechanical circulatory devices have changed the landscape of high-risk PCI in the past decade. The authors believe that, when balancing the risk of vascular complications against the benefit of sufficient haemodynamic support according to existing evidence, implantation of an IABP or an Impella should be considered before patients undergo high-risk PCI.
Both devices require single arterial access and simpler console management than ECMO, without the need for transseptal puncture as required with the TandemHeart. The choice between an IABP and an Impella would depend on multiple factors including device availability, the patient’s haemodynamic requirements and the operator’s preference.
Given the risks associated with emergency MCS implantation during haemodynamic compromise, prophylactic MCS implantation should be considered in selected patients undergoing high-risk PCI.
Ongoing and future research will shed light on unanswered questions in the field including those around the definition of CHIP, patient selection, device selection, evaluation of new devices and timing of support initiation. 
Clinical Perspective
- The emergence of various percutaneous mechanical circulatory support devices has made percutaneous coronary intervention (PCI) a viable option for certain high-risk patients.
- The benefit of periprocedural haemodynamic support should be balanced against the risk of vascular complications when deciding on use of mechanical circulatory support (MCS) in patients undergoing high-risk PCI.
- Given the risk of emergency MCS implantation during haemodynamic compromise, prophylactic MCS implantation should be considered in selected patients undergoing high-risk PCI.
- Ongoing and future research will shed light on unanswered questions in the field, including on the definition of CHIP, patient selection, device selection, evaluation of new devices and the timing of support initiation.